How Do You Separate Oil And Vinegar
Juapaving
Mar 29, 2025 · 6 min read
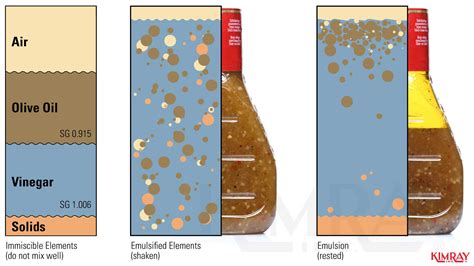
Table of Contents
How Do You Separate Oil and Vinegar? A Comprehensive Guide to Emulsion Separation
Oil and vinegar, a culinary pairing as classic as it is challenging. Their seemingly simple combination masks a fascinating scientific principle: the incompatibility of polar and non-polar substances. This incompatibility is what makes separating them a surprisingly complex (yet achievable!) task. This comprehensive guide delves into the science behind oil and vinegar mixtures, exploring various methods for their separation, and offering tips for achieving the cleanest possible separation.
Understanding the Science: Polarity and Emulsions
The reason oil and vinegar don't mix lies in their molecular structures. Vinegar, primarily water with dissolved acetic acid, is a polar substance. Its molecules have a positive and negative end, allowing them to attract water molecules and other polar substances. Oil, on the other hand, is non-polar. Its molecules lack this positive-negative charge difference, preventing interaction with polar molecules like water.
This fundamental difference leads to the formation of an emulsion when oil and vinegar are combined. An emulsion is a mixture of two immiscible liquids, where one liquid is dispersed as droplets within the other. In the case of oil and vinegar, the oil forms droplets suspended within the vinegar, resulting in a cloudy mixture.
However, simple shaking doesn't create a true solution. The oil and vinegar remain separate entities, held together only temporarily by surface tension and any emulsifying agents present. This is crucial to understanding the various separation techniques.
Methods for Separating Oil and Vinegar
Several methods can effectively separate oil and vinegar, each with varying degrees of effectiveness and practicality. The best method depends on the quantity of the mixture, the desired level of purity, and the available resources.
1. Gravity Separation: The Simplest Approach
This is the most basic and widely known method. It leverages the difference in density between oil and vinegar. Oil is less dense than vinegar, meaning it will float on top.
How to do it:
-
Allow the mixture to stand undisturbed. The longer you wait, the clearer the separation will become. Gravity will gradually pull the denser vinegar downwards, while the oil will rise to the surface. This process can take anywhere from a few minutes to several hours, depending on the viscosity of the oil and the volume of the mixture.
-
Carefully pour off the top layer (oil). Use a slow, steady hand to avoid disturbing the interface between the oil and vinegar layers. A small funnel can help direct the flow of the oil.
-
Optional: Use a pipette or syringe. For more precise separation, especially with smaller quantities, a pipette or syringe can selectively remove the oil layer without disturbing the vinegar.
Limitations: This method is most effective for larger volumes where a clear separation between layers is easily visible. It's also less efficient with highly viscous oils or those containing emulsifiers, which can hinder the separation process.
2. Centrifugation: Speeding Up the Process
A centrifuge uses centrifugal force to accelerate the separation process. By rapidly spinning the oil and vinegar mixture, the heavier vinegar is forced to the bottom, while the lighter oil rises to the top, resulting in faster and more efficient separation.
How to do it:
-
Place the oil and vinegar mixture in a centrifuge tube. Ensure the tubes are properly balanced to avoid vibrations during centrifugation.
-
Spin the centrifuge at a moderate speed. The optimal speed will depend on the volume and viscosity of the mixture. Higher speeds can be used for better separation, but excessive speeds could damage the centrifuge or the sample.
-
Carefully remove the separated layers. After centrifugation, the oil and vinegar layers will be clearly separated, allowing for easier extraction.
Advantages: Centrifugation is significantly faster than gravity separation and is more effective with viscous oils or those containing emulsifiers.
Limitations: Requires access to a centrifuge, which is not commonly available in home settings.
3. Using a Separatory Funnel: Precise Separation
A separatory funnel, also known as a separating funnel, is a laboratory apparatus specifically designed for separating immiscible liquids.
How to do it:
-
Pour the oil and vinegar mixture into the separatory funnel. Ensure the stopcock at the bottom is closed.
-
Allow the mixture to settle. Gravity will separate the oil and vinegar layers.
-
Slowly open the stopcock. Drain the lower (vinegar) layer into a separate container. Close the stopcock just before the oil layer begins to flow out.
-
Collect the top layer (oil). Carefully remove the separatory funnel and pour out the remaining oil layer.
Advantages: Separatory funnels offer precise and efficient separation, particularly useful for smaller quantities of mixture.
Limitations: Requires specialized equipment (separatory funnel).
4. Absorption: For Traces of Oil
If you need to remove the remaining trace amounts of oil from the vinegar after using gravity separation or other methods, absorption techniques can be employed. This often involves using a material that absorbs the oil but not the vinegar.
How to do it:
-
Use absorbent materials. Options include filter paper, activated charcoal, or even bread. The choice depends on the type of oil and the desired level of purity.
-
Filter the vinegar. Slowly pour the vinegar through the absorbent material. The oil will be absorbed, leaving cleaner vinegar.
Limitations: This is not a primary method for separation, but a supplementary technique for final purification.
Factors Affecting Separation
Several factors can influence the effectiveness of oil and vinegar separation:
- Viscosity of the oil: More viscous oils separate more slowly.
- Presence of emulsifiers: Emulsifiers (like lecithin) stabilize emulsions, making separation more challenging.
- Temperature: Temperature affects the viscosity of both oil and vinegar, potentially influencing separation speed.
- Volume of mixture: Larger volumes require more time for gravity separation.
Troubleshooting Common Issues
- Incomplete separation: Insufficient settling time, the presence of emulsifiers, or highly viscous oil could be responsible. Try increasing settling time or using centrifugation.
- Emulsification: Vigorous shaking can create a stable emulsion, hindering separation. Gentle mixing is preferred.
- Cloudy vinegar: Residual oil droplets might remain. Consider using a filter or repeating the separation process.
Conclusion
Separating oil and vinegar is a seemingly simple task that reveals the fascinating world of polarity and emulsion chemistry. While gravity separation offers a readily available method, other techniques, such as centrifugation and using a separatory funnel, provide greater speed and precision. Understanding the science behind the process and employing the appropriate techniques will ensure a clean and effective separation, unlocking the potential of each ingredient for culinary and other applications. Remember to always prioritize safety and use appropriate safety measures when working with laboratory equipment.
Latest Posts
Latest Posts
-
The Amount Of Matter An Object Has
Mar 31, 2025
-
70 As A Product Of Prime Factors
Mar 31, 2025
-
A Ray Has Two Endpoints True Or False
Mar 31, 2025
-
A Substance That Cannot Be Broken Down By Chemical Means
Mar 31, 2025
-
Do Both Prokaryotic And Eukaryotic Cells Have Ribosomes
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Do You Separate Oil And Vinegar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
