Is Oxygen Gas A Pure Substance
Juapaving
Mar 22, 2025 · 5 min read
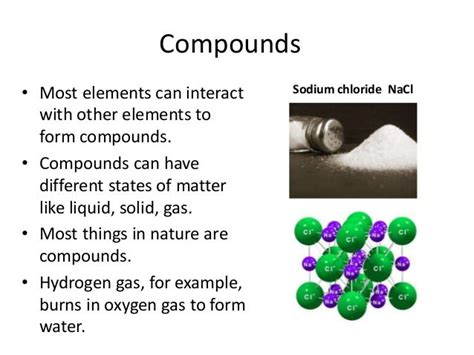
Table of Contents
Is Oxygen Gas a Pure Substance? A Deep Dive into Matter Classification
The question, "Is oxygen gas a pure substance?" might seem simple at first glance. However, understanding the answer requires a deeper dive into the fundamental concepts of chemistry and the classification of matter. This article will explore the definition of a pure substance, examine the properties of oxygen gas, and definitively answer the question while exploring related concepts.
Understanding Pure Substances and Mixtures
Before classifying oxygen gas, let's establish a clear understanding of pure substances and mixtures. Matter, anything that occupies space and has mass, is broadly categorized into two main types: pure substances and mixtures.
Pure Substances: The Building Blocks of Matter
A pure substance is a form of matter that has a constant composition (it's always made up of the same "stuff") and properties throughout the sample. This means that no matter where you take a sample from (assuming it's a homogenous pure substance), its chemical makeup and physical characteristics remain consistent. Pure substances can be further divided into two categories:
-
Elements: These are the fundamental building blocks of matter. They cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). Elements are represented by unique symbols on the periodic table.
-
Compounds: Compounds are formed when two or more different elements combine chemically in fixed proportions. The properties of a compound are distinct from the properties of its constituent elements. For example, water (H₂O) is a compound formed from hydrogen and oxygen; its properties are vastly different from those of hydrogen gas and oxygen gas. Compounds can be broken down into simpler substances through chemical reactions.
Mixtures: A Blend of Substances
In contrast to pure substances, mixtures contain two or more substances that are physically combined but not chemically bonded. Mixtures can have variable compositions, meaning the ratio of the components can change. Mixtures can be further categorized into:
-
Homogeneous Mixtures: These have a uniform composition throughout the sample. You won't see individual components easily. Examples include saltwater, air (a mixture of gases), and sugar dissolved in water.
-
Heterogeneous Mixtures: These have a non-uniform composition; you can visually distinguish the different components. Examples include sand and water, oil and water, and a salad.
The Case of Oxygen Gas (O₂)
Oxygen, in its gaseous form (O₂), is a diatomic molecule, meaning it exists as two oxygen atoms bonded together. This molecular form is crucial for understanding its classification.
Oxygen's Constant Composition and Properties
Oxygen gas (O₂) consistently maintains a fixed ratio of two oxygen atoms per molecule. No matter the source of the oxygen—whether it's from the air, produced by electrolysis of water, or obtained from a commercial gas cylinder—the composition remains the same: O₂. Its physical properties, such as boiling point (-183°C), melting point (-218°C), density, and reactivity, are also constant under given conditions. These consistent properties solidify its classification as a pure substance.
Separating Oxygen: A Pure Substance Test
A key characteristic of a pure substance is its inability to be separated into simpler components by physical means. While methods like fractional distillation can separate components of air (a mixture), these techniques do not alter the chemical composition of the oxygen itself. Oxygen gas extracted from air remains O₂—its chemical identity hasn't changed. To break down oxygen into simpler components (individual oxygen atoms), you would need to employ extreme energy, which is not a typical physical separation process.
Addressing Common Misconceptions
Despite the straightforward nature of its classification, some misconceptions might arise. Let's address a few:
-
Air is not a pure substance: Air is a homogeneous mixture of gases, primarily nitrogen (N₂), oxygen (O₂), argon (Ar), and trace amounts of other gases. While oxygen is a component of air, air itself is not a pure substance due to its variable composition and the presence of multiple substances.
-
Oxygen from different sources is still pure: Oxygen obtained from various sources, such as liquid air separation or electrolysis of water, might contain trace impurities. However, these impurities are typically negligible, and the dominant component remains O₂. The presence of trace impurities does not disqualify it from being a pure substance. The crucial aspect is the overwhelming predominance of O₂, maintaining its consistent composition and properties.
-
Oxygen's isotopic variations: Oxygen exists in three main isotopes: ¹⁶O, ¹⁷O, and ¹⁸O. These isotopes have different numbers of neutrons but the same number of protons. The presence of isotopes does not change the fundamental chemical identity of oxygen, as they behave identically in chemical reactions. Therefore, the presence of different oxygen isotopes within a sample doesn’t prevent oxygen from being classified as a pure substance.
Conclusion: Oxygen Gas is a Pure Substance
Based on the consistent composition, unchanging properties, and the inability to separate it into simpler components through physical means, oxygen gas (O₂) is definitively classified as a pure substance, specifically an element in its molecular form. While it might be found mixed with other substances, such as in air, the oxygen molecule itself remains a fundamental and constant entity. Understanding this distinction highlights the importance of precise definitions and classifications in the world of chemistry. The consistent nature of oxygen gas underscores its crucial role in various biological and industrial processes. Its purity is fundamental to its function, from respiration in living organisms to its applications in welding and other industrial processes.
Latest Posts
Latest Posts
-
Sample Space Of Tossing A Coin 3 Times
Mar 22, 2025
-
Power Factor In An Ac Circuit
Mar 22, 2025
-
How To Find Change In Potential Energy
Mar 22, 2025
-
The Dna Containing Region Of This Bacterial Cell
Mar 22, 2025
-
What Is The Lcm Of 7 And 5
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Oxygen Gas A Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
