Is Koh A Strong Or Weak Base
Juapaving
Mar 24, 2025 · 5 min read
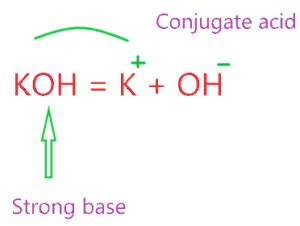
Table of Contents
Is KOH a Strong or Weak Base? A Comprehensive Exploration
Potassium hydroxide (KOH), also known as caustic potash, is a highly alkaline substance frequently used in various industrial and laboratory settings. A crucial aspect of understanding its applications and safety protocols lies in determining its strength as a base. This article will delve into the properties of KOH, explaining why it's definitively classified as a strong base, exploring its dissociation behavior, and examining the implications of its strength in different contexts.
Understanding the Concept of Strong and Weak Bases
Before we dive into the specifics of KOH, let's clarify the fundamental difference between strong and weak bases. The strength of a base is determined by its ability to completely dissociate in water, releasing hydroxide ions (OH⁻).
-
Strong bases: These bases completely ionize in aqueous solutions, meaning nearly all the base molecules break apart into their constituent ions (metal cation and hydroxide anion). This results in a high concentration of OH⁻ ions, leading to a high pH value.
-
Weak bases: These bases only partially ionize in water. A significant portion of the weak base molecules remain undissociated, resulting in a lower concentration of OH⁻ ions and a lower pH compared to a strong base of the same concentration. The equilibrium between the undissociated base and its ions is governed by an equilibrium constant (Kb).
KOH: A Complete Dissociation Story
KOH, being an alkali metal hydroxide, is a classic example of a strong base. When dissolved in water, it undergoes complete dissociation, as represented by the following equation:
KOH(aq) → K⁺(aq) + OH⁻(aq)
This equation shows that one mole of KOH produces one mole of potassium ions (K⁺) and one mole of hydroxide ions (OH⁻). The complete dissociation ensures a high concentration of hydroxide ions, making the solution highly alkaline. This is unlike weak bases which establish an equilibrium between the undissociated base and its ions, resulting in a much lower concentration of OH⁻ ions.
Experimental Evidence Supporting KOH's Strength
Several experimental methods can confirm the strong base nature of KOH.
1. Conductivity Measurements:
Strong electrolytes, such as strong bases, exhibit high electrical conductivity in aqueous solutions due to the high concentration of freely moving ions. KOH solutions show significantly higher conductivity than solutions of weak bases of comparable concentrations, directly demonstrating the near-complete dissociation of KOH into ions.
2. pH Measurements:
A simple pH measurement is a robust indicator of basicity. A concentrated KOH solution will have a very high pH value (typically above 12), reflecting the high concentration of OH⁻ ions produced from its complete dissociation. This contrasts sharply with weak bases, which show much lower pH values even at comparable concentrations.
3. Titration Experiments:
Titration experiments involving KOH demonstrate its strong base nature. A titration curve for a strong acid-strong base titration (e.g., HCl vs. KOH) shows a sharp equivalence point, indicating the complete neutralization of the acid by the base. This sharp equivalence point is characteristic of the reaction between a strong acid and a strong base.
The Implications of KOH's Strength
The fact that KOH is a strong base has significant implications across various applications:
1. Industrial Applications:
-
Soap and Detergent Production: KOH is a crucial component in the saponification process, converting fats and oils into soap. Its strong basicity is essential for effectively breaking down the ester bonds in these molecules.
-
Food Processing: In some food processing applications, KOH acts as a pH regulator or catalyst. Its strong base character helps in controlling the acidity or alkalinity of the final product.
-
Chemical Synthesis: KOH's strong basicity is exploited in numerous chemical synthesis reactions, serving as a powerful deprotonating agent or a catalyst.
-
Wastewater Treatment: KOH contributes to neutralizing acidic waste streams and adjusting the pH levels in various water treatment processes.
2. Laboratory Applications:
-
Acid-Base Titrations: KOH is widely used as a titrant in acid-base titrations, especially when dealing with weak or strong acids. Its strong base nature ensures precise and reliable titration results.
-
Organic Chemistry: KOH's ability to abstract protons is exploited in many organic reactions such as eliminations and condensations.
-
Analytical Chemistry: KOH solutions are used in various analytical techniques for pH adjustments or as a reagent in chemical analysis procedures.
3. Safety Considerations:
The strong base nature of KOH necessitates careful handling and safety precautions. Direct contact with skin or eyes can cause severe burns and irritation. Appropriate personal protective equipment (PPE), including gloves, eye protection, and lab coats, is essential when working with KOH. Spills should be handled with extreme caution, neutralizing the KOH with a weak acid such as acetic acid before cleanup.
Comparison with Weak Bases
Let's highlight the key differences between KOH (a strong base) and a typical weak base, such as ammonia (NH₃):
| Feature | KOH (Strong Base) | NH₃ (Weak Base) |
|---|---|---|
| Dissociation | Complete dissociation in water | Partial dissociation in water |
| Hydroxide Ions | High concentration of OH⁻ ions | Low concentration of OH⁻ ions |
| pH | High pH (typically above 12 for concentrated solutions) | Lower pH compared to KOH at similar concentrations |
| Conductivity | High electrical conductivity in aqueous solution | Lower electrical conductivity compared to KOH solutions |
| Titration Curve | Sharp equivalence point in acid-base titrations | Less sharp equivalence point in acid-base titrations |
| Equilibrium | No significant equilibrium between KOH and ions | Equilibrium exists between NH₃ and NH₄⁺ and OH⁻ ions |
Conclusion: KOH – A Potent Strong Base
In conclusion, KOH undeniably qualifies as a strong base due to its complete dissociation in water, resulting in a high concentration of hydroxide ions. This characteristic underlies its widespread applications in various industrial processes, laboratory procedures, and necessitates careful handling due to its corrosive nature. Understanding the difference between strong and weak bases is crucial for accurately predicting chemical behavior, ensuring safe handling practices, and effectively utilizing KOH in various applications. The experimental evidence, including conductivity measurements, pH readings, and titration experiments, firmly supports its classification as a strong base. Its powerful basicity dictates its diverse applications and highlights the importance of safety procedures when handling this potent chemical.
Latest Posts
Latest Posts
-
99 Rounded To The Nearest Tenth
Mar 28, 2025
-
What Percent Is Equivalent To 3 8
Mar 28, 2025
-
What Is The Greatest Common Factor Of 75
Mar 28, 2025
-
What Is A Subset Of A Real Number
Mar 28, 2025
-
How Many Cm Is 3 5 Inches
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Koh A Strong Or Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
