Is Evaporation Of Water Endothermic Or Exothermic
Juapaving
Mar 22, 2025 · 5 min read
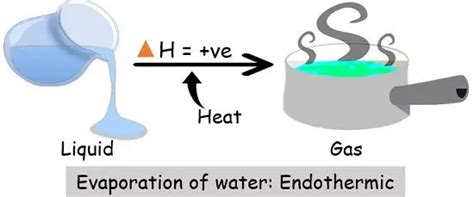
Table of Contents
Is Evaporation of Water Endothermic or Exothermic? A Deep Dive into Phase Transitions
The question of whether evaporation is endothermic or exothermic often arises in chemistry and physics discussions. Understanding this seemingly simple process reveals fascinating insights into the nature of energy transfer at a molecular level. The short answer is: evaporation is an endothermic process. But let's explore the "why" behind this, delving into the intricacies of molecular behavior, enthalpy changes, and the practical implications of this fundamental process.
Understanding Endothermic and Exothermic Reactions
Before diving into the specifics of evaporation, let's establish a clear understanding of endothermic and exothermic reactions. These terms describe the energy changes that occur during a process.
-
Exothermic reactions: These reactions release energy into their surroundings. Think of combustion – burning wood releases heat and light. The system's energy decreases, and the surroundings' energy increases. The enthalpy change (ΔH) for an exothermic reaction is negative.
-
Endothermic reactions: These reactions absorb energy from their surroundings. Melting ice is a prime example; it requires energy from the environment to break the bonds holding the water molecules in a solid structure. The system's energy increases, and the surroundings' energy decreases. The enthalpy change (ΔH) for an endothermic reaction is positive.
The Evaporation Process: A Molecular Perspective
Evaporation is a phase transition where a liquid transforms into a gas. Let's examine this at the molecular level:
Molecular Bonds and Kinetic Energy
Water molecules in a liquid are constantly in motion, possessing kinetic energy. The strength of the intermolecular forces (hydrogen bonds in the case of water) determines the liquid's cohesion. Some molecules possess higher kinetic energy than others.
Escape from the Liquid Phase
For a water molecule to evaporate, it needs enough kinetic energy to overcome the attractive forces holding it within the liquid. These high-energy molecules break free from the surface and enter the gaseous phase. Because only the fastest molecules escape, the average kinetic energy of the remaining liquid molecules decreases. This leads to a decrease in the temperature of the liquid unless heat is continuously supplied.
The Role of Heat Energy
The energy required for the phase transition from liquid to gas is absorbed from the surroundings. This is why evaporation is an endothermic process; it needs a continuous input of energy to sustain the process. This energy is used to break the intermolecular forces binding the molecules in the liquid state, enabling them to transition to the higher-energy gaseous state. Without this energy input, the evaporation process would quickly cease.
Enthalpy of Vaporization: Quantifying the Energy Change
The enthalpy of vaporization (ΔH<sub>vap</sub>) is the amount of heat energy required to vaporize one mole of a liquid at its boiling point under constant pressure. For water, this value is approximately 40.7 kJ/mol. This positive value confirms the endothermic nature of evaporation.
It's important to note that the enthalpy of vaporization is temperature-dependent. While the value of 40.7 kJ/mol is for water at its boiling point (100°C), the energy required for evaporation at lower temperatures will be slightly higher. This is because at lower temperatures, a greater proportion of the absorbed energy goes into increasing the kinetic energy of the water molecules rather than solely breaking intermolecular forces.
Practical Implications of Endothermic Evaporation
The endothermic nature of evaporation has significant real-world implications:
Cooling Effects
Evaporation causes a cooling effect because it absorbs heat energy from its surroundings. This is why sweating cools the human body; the evaporation of perspiration absorbs heat from the skin, lowering its temperature. Similarly, evaporative coolers (swamp coolers) utilize the endothermic nature of water evaporation to cool air.
Weather Patterns
Evaporation plays a crucial role in weather patterns. The evaporation of water from oceans, lakes, and rivers provides the moisture for cloud formation and precipitation. The energy absorbed during evaporation is later released during condensation, driving atmospheric circulation and weather systems.
Industrial Processes
Many industrial processes utilize the endothermic nature of evaporation, for example, in distillation and drying processes. Understanding the energy requirements for evaporation is crucial for designing and optimizing these processes.
Distinguishing Evaporation from Boiling
While both evaporation and boiling involve the phase transition of liquid to gas, they differ in several key aspects:
-
Temperature: Evaporation occurs at any temperature below the boiling point, while boiling occurs at the boiling point of the liquid.
-
Location: Evaporation takes place only at the surface of the liquid, while boiling occurs throughout the liquid's bulk.
-
Rate: Boiling generally occurs at a faster rate than evaporation under normal atmospheric conditions.
Factors Affecting the Rate of Evaporation
Several factors influence the rate of evaporation:
-
Temperature: Higher temperatures lead to faster evaporation due to increased kinetic energy of molecules.
-
Surface area: Larger surface areas allow more molecules to escape simultaneously, increasing the rate of evaporation.
-
Air movement: Wind or air currents remove water vapor from above the liquid's surface, allowing more molecules to escape, thereby accelerating evaporation.
-
Humidity: High humidity slows evaporation because the air is already saturated with water vapor, reducing the driving force for further evaporation.
Conclusion: Evaporation as a Fundamental Endothermic Process
In conclusion, the evaporation of water is unequivocally an endothermic process. This fundamental process underpins numerous natural phenomena and technological applications. Understanding the molecular mechanisms, energy changes, and influencing factors associated with evaporation is crucial in fields ranging from meteorology and climatology to chemical engineering and biology. Its significant cooling effect has far-reaching consequences, from regulating body temperature to influencing global weather patterns. The positive enthalpy of vaporization serves as a clear quantitative indicator of the energy absorption involved in this essential phase transition. Further exploration into this process reveals the rich interplay between energy, molecules, and macroscopic phenomena that govern our world.
Latest Posts
Latest Posts
-
What Are Characteristics Of Covalent Compounds
Mar 22, 2025
-
Calculate The Molecular Mass Of H2co3
Mar 22, 2025
-
The Study Of The Function Of Tissues Is Called
Mar 22, 2025
-
Which Is Not A Property Of Living Being
Mar 22, 2025
-
In Which Two Hemispheres Is Australia Located
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Evaporation Of Water Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
