Is Evaporation An Endothermic Or Exothermic Process
Juapaving
Mar 19, 2025 · 6 min read
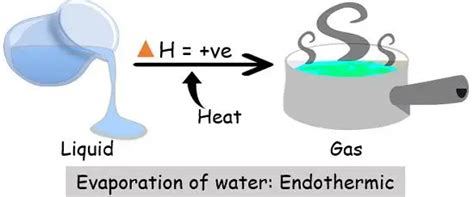
Table of Contents
Is Evaporation an Endothermic or Exothermic Process? A Deep Dive
Evaporation, a ubiquitous process shaping our climate and crucial to various industrial applications, often sparks the question: is it endothermic or exothermic? Understanding this fundamental aspect is key to grasping its implications in various scientific fields and everyday life. This comprehensive article will delve into the intricacies of evaporation, examining its thermodynamic nature, exploring the underlying mechanisms, and providing real-world examples to solidify understanding.
Understanding Endothermic and Exothermic Processes
Before diving into the specifics of evaporation, let's clarify the terms "endothermic" and "exothermic." These terms describe the energy changes associated with a process:
-
Endothermic Process: An endothermic process absorbs heat from its surroundings. The system's energy increases, resulting in a decrease in the surrounding temperature. Think of it like a sponge soaking up water; the sponge (the system) gains energy, and the water (the surroundings) loses energy.
-
Exothermic Process: An exothermic process releases heat into its surroundings. The system's energy decreases, leading to an increase in the surrounding temperature. Imagine a burning candle; the candle (the system) loses energy as heat and light are released into the surrounding air.
The Nature of Evaporation: An Endothermic Process
Evaporation is unequivocally an endothermic process. This means it requires energy input to occur. The energy is absorbed to overcome the intermolecular forces holding the liquid molecules together, allowing them to transition into the gaseous phase.
The Molecular Dance: Breaking Intermolecular Bonds
Liquid water, for instance, consists of water molecules (H₂O) held together by relatively strong hydrogen bonds. These bonds require a significant amount of energy to break. During evaporation, some molecules at the liquid's surface possess sufficient kinetic energy to overcome these attractive forces and escape into the gaseous phase as water vapor. This energy is absorbed from the surroundings, hence the endothermic nature of the process.
Latent Heat of Vaporization: The Energy Cost of Evaporation
The amount of energy required to evaporate a substance is quantified as its latent heat of vaporization. This is the energy needed to change one unit mass of a substance from a liquid to a gas at a constant temperature. Water has a relatively high latent heat of vaporization, meaning a significant amount of energy is required to convert liquid water into water vapor. This high value contributes to water's crucial role in regulating Earth's temperature.
Real-World Examples of Evaporation's Endothermic Nature
Numerous everyday occurrences demonstrate evaporation's endothermic nature:
-
Sweating: Our bodies use sweating as a cooling mechanism. As sweat evaporates from our skin, it absorbs heat from our body, lowering our skin temperature. This is why we feel cooler after sweating, even in a humid environment. In a dry environment, the evaporation rate is higher and the cooling effect is more pronounced.
-
Cooling Drinks: On a hot day, a wet cloth placed on a bottle of drink will cool it down. The evaporating water absorbs heat from the bottle, thus lowering its temperature. This principle is employed in evaporative coolers, commonly used in arid climates.
-
Drying Clothes: Wet clothes dry because the water molecules in the fabric absorb energy from the surrounding air and evaporate. This process leaves the clothes feeling cooler and eventually dry.
-
Formation of Clouds: The formation of clouds involves evaporation from water bodies, like oceans and lakes. The sun's energy drives evaporation, converting liquid water into water vapor. This water vapor then rises, cools, and condenses to form clouds. The initial evaporation stage is, once again, an endothermic process.
Factors Affecting Evaporation Rate: A Deeper Look
Several factors influence the rate of evaporation:
-
Temperature: Higher temperatures increase the kinetic energy of liquid molecules, leading to faster evaporation. More molecules have the energy to overcome intermolecular forces and escape into the gaseous phase.
-
Surface Area: A larger surface area exposes more liquid molecules to the atmosphere, enhancing the rate of evaporation. This is why spreading laundry on a clothesline facilitates faster drying compared to bundling it together.
-
Humidity: High humidity (high concentration of water vapor in the air) reduces evaporation rates. The air already contains a significant amount of water vapor, making it less likely for more water molecules to transition from the liquid to the gaseous phase.
-
Wind: Wind accelerates evaporation by constantly removing water vapor from the vicinity of the liquid surface. This reduces the concentration of water vapor in the air, allowing more molecules to evaporate.
-
Atmospheric Pressure: Lower atmospheric pressure reduces the resistance that the air exerts on evaporating molecules, leading to faster evaporation. This is why evaporation rates are higher at higher altitudes.
Evaporation and Climate: A Vital Role
Evaporation plays a crucial role in shaping our climate and weather patterns:
-
The Water Cycle: Evaporation is the primary driver of the water cycle. Solar energy drives evaporation from oceans, lakes, and rivers, creating water vapor that rises into the atmosphere. This water vapor eventually condenses to form clouds, which then release precipitation, replenishing water bodies and maintaining the balance of Earth’s water.
-
Temperature Regulation: The high latent heat of vaporization of water means that evaporation effectively absorbs a large amount of heat energy from the environment. This helps to regulate global and regional temperatures, preventing extreme temperature fluctuations.
-
Ocean Currents: Evaporation from oceans contributes to ocean salinity and influences ocean currents, which play a major role in distributing heat around the globe.
Implications in Various Fields
Understanding the endothermic nature of evaporation is crucial in several industrial and scientific applications:
-
Refrigeration: Refrigerators utilize evaporation as a cooling mechanism. A refrigerant liquid evaporates inside the refrigerator, absorbing heat from the interior, thus cooling the contents.
-
Air Conditioning: Similar to refrigeration, air conditioning systems use evaporation of refrigerants to cool the air.
-
Desalination: Desalination plants use evaporation to separate salt from seawater. The evaporated water is then condensed and collected as freshwater.
-
Power Generation: Some power plants utilize the evaporation of water to generate electricity.
Conclusion: Evaporation – An Endothermic Process with Far-Reaching Consequences
In conclusion, evaporation is undeniably an endothermic process, requiring energy input to overcome intermolecular forces and transition liquid molecules into the gaseous phase. This fundamental characteristic has profound implications for various aspects of our lives, from the cooling effect of sweat to the crucial role it plays in the water cycle and climate regulation. Understanding the factors that influence evaporation rates and its thermodynamic nature is crucial for numerous applications in science, engineering, and everyday life. The implications are far-reaching, impacting weather patterns, climate stability, and technological advancements. Its endothermic nature is not merely an abstract scientific concept, but a driving force shaping the world around us.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 7 And 9
Mar 19, 2025
-
A Diagram Of A Compound Microscope
Mar 19, 2025
-
350 Square Meters To Square Feet
Mar 19, 2025
-
What Is The Scientific Name For A Human
Mar 19, 2025
-
5 Letter Words That End With On
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Evaporation An Endothermic Or Exothermic Process . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
