Is Evaporating Water Endothermic Or Exothermic
Juapaving
Mar 24, 2025 · 5 min read
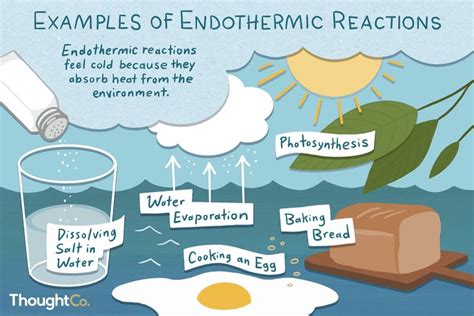
Table of Contents
Is Evaporating Water Endothermic or Exothermic? A Deep Dive into Thermodynamics
Understanding whether evaporating water is endothermic or exothermic is crucial for grasping fundamental concepts in thermodynamics and chemistry. This process, seemingly simple, reveals much about energy transfer and phase changes. This article will delve into the specifics, providing a detailed explanation accessible to both beginners and those seeking a deeper understanding.
Understanding Endothermic and Exothermic Processes
Before tackling the specifics of evaporating water, let's establish a clear definition of endothermic and exothermic processes. These terms describe the energy exchange between a system (in this case, the water) and its surroundings.
-
Exothermic Processes: These processes release energy into their surroundings. The system loses energy, leading to a decrease in its internal energy. Think of combustion – the burning of fuel releases heat into the environment. The system (the fuel) loses energy, and the surroundings gain energy. This is often accompanied by an increase in temperature.
-
Endothermic Processes: These processes absorb energy from their surroundings. The system gains energy, leading to an increase in its internal energy. A classic example is melting ice. Energy from the surroundings (the air) is absorbed to break the bonds holding the water molecules in the ice lattice. The system (the ice) gains energy, and the surroundings lose energy. This often results in a decrease in temperature.
The Evaporation Process: A Molecular Perspective
Evaporation is a phase transition where a liquid transforms into a gas. Let's examine this process at a molecular level to understand the energy dynamics involved.
Breaking Intermolecular Forces
Water molecules are held together by relatively strong intermolecular forces, primarily hydrogen bonds. These bonds are responsible for water's unique properties, such as its high boiling point and surface tension. For a water molecule to escape the liquid phase and enter the gaseous phase (vapor), it must overcome these attractive forces. This requires an input of energy.
Kinetic Energy and Escape Velocity
Water molecules possess kinetic energy, which is the energy of motion. At any given temperature, the molecules move at various speeds. Only those molecules with sufficient kinetic energy can overcome the intermolecular forces and escape the liquid surface. These molecules have reached what's often called "escape velocity" for the liquid.
The Energy Source: The Surroundings
The energy needed to break the intermolecular bonds and allow molecules to evaporate comes from the surroundings. The surroundings could be the air, another object, or even the water itself. As molecules with high kinetic energy escape, the average kinetic energy of the remaining water molecules decreases. This decrease in average kinetic energy manifests as a drop in temperature. This temperature drop is often noticeable, especially with large amounts of evaporating water.
Why Evaporating Water is Endothermic
Based on our analysis, it becomes clear that evaporating water is an endothermic process. The system (the water) absorbs energy from its surroundings to overcome the intermolecular forces and transition into the gaseous phase. The energy absorbed is used to increase the kinetic energy of the escaping molecules, allowing them to overcome the attractive forces and enter the vapor phase.
Practical Examples and Applications of Endothermic Evaporation
The endothermic nature of evaporation has numerous practical applications and implications in everyday life and various industries:
1. Cooling Effects:
-
Sweating: Our bodies utilize evaporative cooling to regulate temperature. Sweat, primarily water, evaporates from our skin, absorbing heat in the process and leaving us feeling cooler.
-
Swamp Coolers (Evaporative Coolers): These devices use the endothermic nature of evaporation to cool air. Water is evaporated, absorbing heat from the surrounding air, leading to a temperature decrease.
-
Refrigeration: Although refrigeration involves more complex cycles than simple evaporation, the initial step often involves evaporating a refrigerant liquid, absorbing heat in the process.
2. Environmental Impacts:
-
Water Cycle: Evaporation plays a crucial role in the Earth's water cycle. The sun's energy drives evaporation, transferring vast amounts of heat energy into the atmosphere.
-
Climate Regulation: Evaporation and condensation influence weather patterns and climate regulation through processes like cloud formation and precipitation.
-
Ecosystems: Evaporation from bodies of water and plants impacts the temperature and humidity of local ecosystems.
3. Industrial Applications:
-
Drying Processes: Many industrial processes rely on evaporation to remove water from materials, like drying clothes, food, or wood.
-
Crystallization: In some crystallization processes, controlled evaporation is used to increase solute concentration and promote crystal formation.
-
Distillation: Evaporation is a key component of distillation, which separates liquids based on their boiling points.
Factors Affecting the Rate of Evaporation
Several factors influence the rate at which water evaporates, all directly related to the energy transfer involved:
-
Temperature: Higher temperatures increase the kinetic energy of water molecules, allowing more molecules to reach escape velocity and evaporate faster.
-
Surface Area: A larger surface area exposes more water molecules to the atmosphere, increasing the evaporation rate.
-
Humidity: High humidity reduces the rate of evaporation because the air is already saturated with water vapor. There's less space for more water molecules to enter the gaseous phase.
-
Air Movement: Air movement (wind) removes water vapor from the surface of the water, allowing more molecules to evaporate.
-
Atmospheric Pressure: Lower atmospheric pressure reduces the resistance to evaporation, leading to a faster rate.
Misconceptions about Evaporation
It's important to clarify some common misconceptions surrounding evaporation:
-
Evaporation is not boiling: While both involve a phase transition from liquid to gas, boiling occurs at a specific temperature (the boiling point) and involves the formation of vapor bubbles within the liquid. Evaporation occurs at temperatures below the boiling point and only at the liquid's surface.
-
Evaporation doesn't always lead to a decrease in temperature: While evaporation is endothermic, a decrease in temperature isn't always observed. If the surrounding environment can readily supply the energy needed for evaporation, the temperature may remain constant or even increase slightly.
Conclusion
In summary, evaporating water is demonstrably an endothermic process. It requires energy input from the surroundings to break the intermolecular forces holding water molecules together and allow them to transition into the gaseous phase. This understanding has significant implications across various fields, from everyday life to industrial applications and climate science. Appreciating the energy dynamics involved provides a deeper appreciation of the fundamental principles of thermodynamics and the significance of phase transitions in nature. Further exploration of these concepts can unlock a richer understanding of the world around us.
Latest Posts
Latest Posts
-
Oxidation Reduction Reactions In Cellular Respiration
Mar 26, 2025
-
What Direction Does Dna Polymerase Read
Mar 26, 2025
-
What Is 25 Cm In Inches
Mar 26, 2025
-
Which Of The Following Statements Describes The Process Of Globalization
Mar 26, 2025
-
What Are The Greatest Common Factors Of 48
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Evaporating Water Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
