Is Distilled Water An Acid Or Base
Juapaving
Mar 10, 2025 · 5 min read
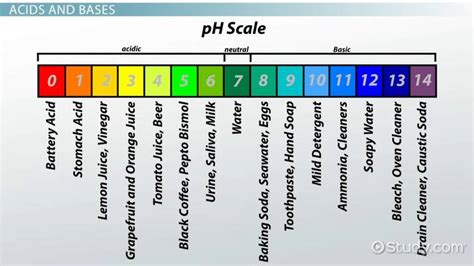
Table of Contents
Is Distilled Water an Acid or a Base? Understanding pH and Purity
Distilled water, often touted for its purity, sparks a common question: is it an acid or a base? The short answer is neither. Distilled water is neutral, meaning it has a pH of approximately 7.0 at 25°C (77°F). However, a deeper understanding requires exploring the concepts of pH, acidity, basicity, and the subtle nuances of water purity. This comprehensive guide will delve into these aspects, addressing common misconceptions and providing a clear picture of distilled water's chemical nature.
Understanding pH: The Measure of Acidity and Basicity
Before we classify distilled water, let's clarify the pH scale. The pH scale is a logarithmic scale ranging from 0 to 14, measuring the concentration of hydrogen ions (H⁺) in a solution. A lower pH value (below 7) indicates a higher concentration of H⁺ ions, signifying an acidic solution. Conversely, a higher pH value (above 7) signifies a lower concentration of H⁺ ions and a higher concentration of hydroxide ions (OH⁻), indicating a basic (or alkaline) solution. A pH of 7 represents a neutral solution, where the concentrations of H⁺ and OH⁻ ions are equal.
The Role of Hydrogen and Hydroxide Ions
Pure water undergoes a process called self-ionization, where a small fraction of water molecules dissociate into H⁺ and OH⁻ ions. This is represented by the following equilibrium equation:
2H₂O ⇌ H₃O⁺ + OH⁻
While this dissociation is minimal, it's crucial in determining water's pH. In pure water, the concentration of both H⁺ and OH⁻ ions is equal, resulting in a neutral pH of 7.
The Purity of Distilled Water and its Impact on pH
The neutrality of distilled water is directly linked to its purity. The distillation process involves boiling water and collecting the condensed steam. This effectively separates water molecules from most dissolved impurities, including minerals, salts, and gases. These impurities, often acidic or basic, can significantly affect the pH of water.
Impurities and pH Alterations
Minerals: Water naturally contains various minerals, like calcium and magnesium carbonates, which can increase its pH, making it slightly alkaline. The distillation process removes these minerals, preventing any significant pH shift towards basicity.
Gases: Dissolved gases like carbon dioxide (CO₂) can react with water to form carbonic acid (H₂CO₃), slightly lowering the pH. Distillation removes dissolved CO₂, preventing the formation of carbonic acid and maintaining neutrality.
Acids and Bases: Other dissolved acids or bases in the source water, such as from industrial pollution or agricultural runoff, can drastically alter the water's pH. Distillation effectively removes these contaminants, ensuring the distilled water maintains a neutral pH.
Factors Affecting the pH of Distilled Water
While ideally distilled water should have a pH of 7, several factors can subtly influence its pH:
-
Carbon Dioxide Absorption: Even after distillation, distilled water can absorb CO₂ from the atmosphere, slightly lowering its pH. This effect is typically minor, but it can be noticeable over time, especially if the water is stored in an open container.
-
Container Material: The material of the storage container can also play a minor role. Certain plastics or metals might leach ions into the water, slightly altering its pH. Using high-quality, inert containers minimizes this risk.
-
Temperature: Temperature affects the self-ionization of water, slightly influencing its pH. The pH of 7 is specifically at 25°C; variations in temperature will result in slight changes.
Common Misconceptions about Distilled Water and pH
Several misconceptions surround distilled water and its pH:
-
Distilled water is completely pure: While distillation significantly increases purity, it's not a perfect process. Trace amounts of volatile impurities might remain.
-
Distilled water is sterile: While distillation kills most microorganisms, it doesn't guarantee sterility. Contamination can occur after the distillation process.
-
The pH of distilled water is always exactly 7: As discussed, various factors can cause slight variations from the ideal pH of 7.
Applications of Distilled Water Utilizing its Neutral pH
The neutral pH of distilled water makes it suitable for various applications where maintaining a specific pH is crucial:
-
Laboratory experiments: Distilled water is widely used in laboratories to prepare solutions and conduct experiments where the presence of impurities could interfere with results. Its neutral pH ensures that the solutions prepared remain within the intended parameters, preventing false readings or unexpected reactions.
-
Medical applications: In some medical applications, where the pH of a solution is critical, distilled water is preferred to avoid any alteration caused by impurities.
-
Aquariums: Distilled water can be used to top up aquariums, provided minerals and other necessary substances are added appropriately. Using distilled water prevents the addition of unwanted minerals or other contaminants which could disrupt the delicate balance of the aquarium's environment.
-
Industrial processes: In several industrial processes, the purity and neutral pH of distilled water are essential to avoid altering the final product’s properties or introducing unwanted substances.
-
Battery applications: Some batteries require distilled water to function properly. Impurities could damage the battery, whereas distilled water's purity and neutral pH allow for optimal performance.
Conclusion: Distilled Water's Neutral Nature
In conclusion, distilled water is essentially neutral, having a pH of approximately 7.0. This neutrality is a direct result of its high purity, achieved through the removal of impurities that could alter its pH. While slight variations can occur due to external factors, the essentially neutral pH of distilled water makes it a valuable resource in numerous applications requiring precise pH control. Understanding the nuances of pH and water purity is essential for appreciating the specific benefits of distilled water in its diverse applications.
Latest Posts
Latest Posts
-
Which Organelle Is Critical For Cell Division
Mar 10, 2025
-
What Is The Least Common Multiple Of 5 And 11
Mar 10, 2025
-
What Is The Purpose Of Petals On A Flower
Mar 10, 2025
-
What Are The Organelles Found Only In Plant Cells
Mar 10, 2025
-
Is 20 A Multiple Of 3
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Distilled Water An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
