Is Chlorine A Metal Nonmetal Or Metalloid
Juapaving
Mar 31, 2025 · 5 min read
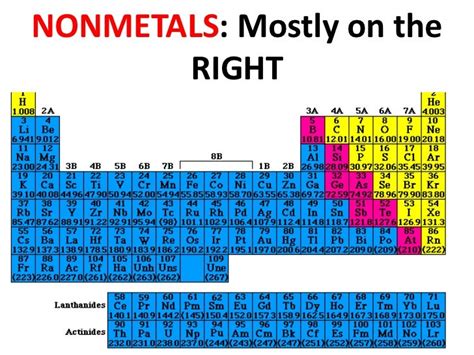
Table of Contents
Is Chlorine a Metal, Nonmetal, or Metalloid? A Deep Dive into Chemical Properties
Chlorine, a ubiquitous element found in everyday life, often sparks curiosity about its fundamental nature. Is it a metal, a nonmetal, or a metalloid? Understanding its classification requires delving into its chemical properties and behavior. This article will explore the characteristics that definitively place chlorine firmly within the nonmetal category, clarifying any confusion and providing a comprehensive overview of its unique attributes.
Defining Metals, Nonmetals, and Metalloids
Before classifying chlorine, it's crucial to establish clear definitions for each category:
Metals
Metals are generally characterized by their:
- High electrical conductivity: They readily conduct electricity due to the ease of electron movement.
- High thermal conductivity: They efficiently transfer heat.
- Malleability and ductility: They can be hammered into sheets (malleability) and drawn into wires (ductility).
- Metallic luster: They possess a shiny appearance.
- Low ionization energy: They easily lose electrons.
- Solid state at room temperature (except mercury): Most exist as solids under normal conditions.
Nonmetals
Nonmetals, in contrast, exhibit:
- Poor electrical conductivity: They are generally poor conductors of electricity.
- Poor thermal conductivity: They do not transfer heat efficiently.
- Brittleness: They tend to be brittle and shatter easily.
- Lack of metallic luster: They often lack a shiny appearance.
- High ionization energy: They strongly resist losing electrons.
- Varied physical states at room temperature: They can exist as solids, liquids, or gases.
Metalloids (Semimetals)
Metalloids possess properties intermediate between metals and nonmetals. They exhibit a mixture of characteristics, making their classification less straightforward. Their conductivity, for instance, is often somewhere between that of metals and nonmetals, and they may display some metallic luster but lack the malleability and ductility of true metals.
Chlorine's Chemical Properties: A Case for Nonmetal Classification
Chlorine's position on the periodic table, in Group 17 (also known as the halogens), is a strong indicator of its nonmetallic nature. Let's examine the specific properties that confirm its classification:
1. Electrical and Thermal Conductivity: Poor Conductors
Chlorine, in its gaseous state at room temperature, is a very poor conductor of both electricity and heat. This contrasts sharply with the high conductivity observed in metals. The electrons in chlorine are tightly bound to their atoms, hindering their movement and preventing the efficient flow of charge or heat.
2. Physical State and Appearance: Gas at Room Temperature
At room temperature and standard pressure, chlorine exists as a yellow-green gas. This gaseous state is atypical for metals, which are predominantly solid at room temperature. Furthermore, chlorine lacks the characteristic metallic luster; it doesn't have the shiny appearance associated with metals.
3. Ionization Energy: High Resistance to Electron Loss
Chlorine exhibits a high ionization energy. This means that it strongly resists losing electrons. It requires a significant amount of energy to remove an electron from a chlorine atom. This high ionization energy is a hallmark of nonmetals, which tend to gain electrons rather than lose them to achieve a stable electron configuration.
4. Chemical Reactivity: Highly Reactive Nonmetal
Chlorine is a highly reactive nonmetal, readily gaining an electron to form the chloride ion (Cl⁻). This high reactivity is a consequence of its electron configuration; it needs only one electron to complete its outermost electron shell, making it highly electronegative (a strong tendency to attract electrons). This characteristic is a key feature of many nonmetals. This tendency to gain electrons is showcased in its reactions with metals, forming ionic compounds like sodium chloride (NaCl), commonly known as table salt.
5. Formation of Covalent Bonds: Sharing, Not Losing, Electrons
While chlorine readily gains electrons to form ionic compounds with metals, it can also form covalent bonds with other nonmetals. In covalent bonding, atoms share electrons rather than transferring them completely, as seen in ionic bonding. This ability to share electrons further reinforces its nonmetal classification. Examples of covalent chlorine compounds include hydrogen chloride (HCl) and carbon tetrachloride (CCl₄).
6. Brittle Solid State: Lack of Malleability and Ductility
When cooled to below its boiling point, chlorine becomes a solid. However, it is brittle, lacking the malleability and ductility of metals. It will fracture under stress rather than deform as metals do.
Comparing Chlorine to Metalloids: Clear Distinctions
While some elements exhibit properties that blend those of metals and nonmetals, chlorine's properties firmly place it in the nonmetal category. Let's compare its characteristics to those of metalloids to highlight the distinctions:
| Feature | Chlorine (Nonmetal) | Metalloids (e.g., Silicon, Germanium) |
|---|---|---|
| Electrical Conductivity | Very poor | Intermediate (semiconductors) |
| Thermal Conductivity | Poor | Intermediate |
| Physical State | Gas (at room temperature) | Solid (at room temperature) |
| Luster | Absent | Can have some metallic luster |
| Ionization Energy | High | Varies, generally intermediate |
| Malleability/Ductility | Absent | Limited or absent |
| Reactivity | High (gains electrons easily) | Varies |
As the table demonstrates, chlorine's properties diverge significantly from those of metalloids. The poor conductivity, gaseous state at room temperature, high ionization energy, and high reactivity are all definitive characteristics of a nonmetal.
Conclusion: Chlorine's Definitive Nonmetal Status
In conclusion, there is no ambiguity in classifying chlorine. Its chemical properties, encompassing poor electrical and thermal conductivity, gaseous state at room temperature, high ionization energy, high reactivity, ability to form both ionic and covalent bonds, and brittle solid form, all strongly support its classification as a nonmetal. Any suggestion that chlorine is a metal or metalloid is incorrect based on its established and well-understood chemical behavior. Its position within the halogen group further reinforces this classification. Therefore, understanding its nonmetal nature is essential to grasping its role in various chemical reactions and applications.
Latest Posts
Latest Posts
-
Endocrine Glands Are Often Called Ducted Glands True False
Apr 01, 2025
-
Where Does Sound Waves Travel Fastest
Apr 01, 2025
-
How Many Grams Is 5 Kg
Apr 01, 2025
-
Newtons First Law Examples In Everyday Life
Apr 01, 2025
-
15 4 As A Mixed Number
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Chlorine A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
