Is Butter Melting A Chemical Or Physical Change
Juapaving
Mar 31, 2025 · 6 min read
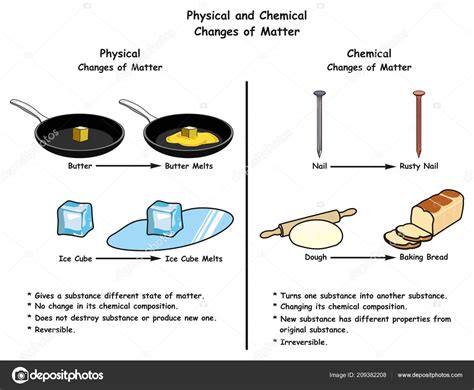
Table of Contents
Is Melting Butter a Chemical or Physical Change? A Deep Dive
The simple act of melting butter seems straightforward enough, but delving into the science behind it reveals a fascinating interplay of physical and chemical processes. While the immediate observation suggests a physical change – a change in state from solid to liquid – a closer examination reveals subtle chemical transformations that occur alongside the primary physical alteration. This article will explore the intricacies of butter melting, differentiating between the physical and chemical changes involved, and addressing common misconceptions.
Understanding Physical Changes
A physical change alters the form or appearance of a substance without changing its chemical composition. Think about cutting paper, dissolving sugar in water, or boiling water. In each case, the substance remains fundamentally the same; it's just in a different form. The molecules themselves don't undergo any fundamental alteration. Reversibility is often a key characteristic of physical changes. For example, you can freeze liquid water back into ice, demonstrating that the process is reversible.
In the context of melting butter, the primary change is undoubtedly physical. The solid butter, consisting of a complex mixture of fats, water, and milk solids, transitions to a liquid state upon heating. The molecules of fat (primarily triglycerides) are simply overcoming their intermolecular forces, allowing them to move more freely and thus changing from a rigid solid structure to a more fluid liquid one. This phase transition is driven by the increased kinetic energy of the molecules as heat is applied. You can solidify the melted butter back into a solid by cooling it, further emphasizing the physical nature of this transformation.
Key Characteristics of Butter's Physical Change During Melting:
- Change in state: Solid butter transforms into liquid butter.
- No new substance formed: The chemical composition of the butter remains largely unchanged.
- Reversibility: The liquid butter can be solidified back into solid butter through cooling.
- Melting point: Butter doesn't melt at a single, sharp temperature, exhibiting a melting range due to its complex composition. This is a characteristic feature of mixtures, not pure substances.
- Changes in physical properties: Viscosity, density, and other physical properties change significantly, but the fundamental chemical building blocks remain the same.
The Subtle Chemical Changes in Melting Butter
While the primary change in melting butter is physical, the process isn't entirely devoid of chemical transformations. These chemical changes are often subtle and occur alongside the physical changes, sometimes at a much slower rate.
1. Hydrolysis of Milk Fats:
Butter contains milk fat, triglycerides which are esters of glycerol and fatty acids. At elevated temperatures, a slow hydrolysis reaction can occur. Hydrolysis is a chemical reaction where water molecules react with the ester bonds in triglycerides, breaking them down into glycerol and fatty acids. This process is accelerated by higher temperatures and prolonged heating. While not significant during the rapid melting process, prolonged heating of butter, as in cooking, will noticeably increase the rate of this reaction.
2. Oxidation of Fatty Acids:
Butter contains unsaturated fatty acids, which are susceptible to oxidation. Oxidation is a chemical reaction where fatty acids react with oxygen, often catalyzed by heat or light. This oxidation process leads to the formation of various compounds, including aldehydes, ketones, and peroxides, contributing to rancidity and off-flavors. The extent of oxidation increases with prolonged heating and exposure to air. While melting butter briefly might not significantly alter the fatty acid profiles, prolonged exposure to high temperatures accelerates this process.
3. Maillard Reaction (at higher temperatures):
At significantly higher temperatures, beyond typical melting points, the Maillard reaction can occur. This is a complex chemical reaction between amino acids and reducing sugars that produces hundreds of different compounds, responsible for the characteristic browning and flavor development in cooked foods. This is most apparent when butter is used for cooking or sautéing, rather than simply melting it. The Maillard reaction is a significant chemical change that contributes to the flavors and aromas of cooked food but is not a primary feature of simple butter melting.
Differentiating Physical and Chemical Changes: A Closer Look
To better understand the distinction, let's consider the following:
-
Physical Changes in Melting Butter: Changes in state (solid to liquid), changes in viscosity, changes in density. These are all reversible processes. If you cool the melted butter, you retrieve the solid form, practically unchanged in chemical composition.
-
Chemical Changes in Melting Butter (during prolonged heating or at high temperatures): Hydrolysis of milk fats, oxidation of unsaturated fatty acids, Maillard reaction (at higher temperatures). These chemical changes are often irreversible and result in the formation of new compounds, altering the overall chemical composition and potentially impacting the taste, smell, and other properties of the butter.
The Importance of Temperature and Time
The extent of chemical changes during butter melting is highly dependent on both the temperature and the duration of heating.
-
Low Temperatures and Short Times: At low temperatures and short periods, the primary process is a physical change, with minimal chemical alterations.
-
High Temperatures and Long Times: At higher temperatures and prolonged periods, chemical changes become increasingly significant, eventually dominating the process and resulting in noticeable alterations in the butter's characteristics, including changes in color, aroma, and taste.
Practical Implications and Misconceptions
Understanding the nuances of butter melting has practical implications for cooking and food science. The difference between a physical and a chemical change dictates how we use butter in different culinary applications.
-
Clarified Butter: The process of making clarified butter involves melting butter and separating the milk solids from the fat. While some chemical changes might occur during this process, the primary change is a separation of components, which is a physical separation, resulting in clarified butter that has a higher smoke point.
-
Cooking with Butter: When butter is used for cooking at high temperatures, the chemical changes become more pronounced. The Maillard reaction and fat oxidation contribute significantly to the development of flavors and aromas but also increase the risk of producing potentially harmful compounds.
-
Common Misconception: Many people mistakenly believe that all melting processes are purely physical. However, many instances of melting involve subtle chemical reactions occurring alongside the physical change of state. The extent of these chemical changes can vary greatly depending on the specific substance and the conditions under which the melting occurs.
Conclusion
The melting of butter is a fascinating example of how physical and chemical changes can occur simultaneously. While the primary change is undeniably a physical transition from a solid to a liquid state, subtle chemical alterations can take place, particularly at higher temperatures or over extended periods. Understanding this interplay of physical and chemical processes is essential for appreciating the complexities of cooking and the transformation of food ingredients. The key takeaway is to recognize that while melting often appears to be a simple physical change, a deeper analysis often reveals subtle but important concurrent chemical reactions. The extent of these chemical changes is heavily influenced by the specific conditions – primarily temperature and duration of heating.
Latest Posts
Latest Posts
-
How Many Particles Are In One Mole Of A Substance
Apr 01, 2025
-
What Are Aerial Parts Of A Plant
Apr 01, 2025
-
What Is The Difference Between Absorption And Emission Spectrum
Apr 01, 2025
-
What Is The Waste Product Of Photosynthesis
Apr 01, 2025
-
Sound Travels Fastest In Which Medium
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Butter Melting A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
