Is Air A Mixture Or Pure Substance
Juapaving
Mar 25, 2025 · 6 min read
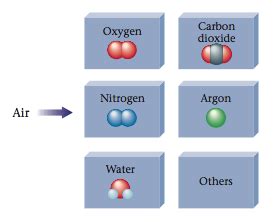
Table of Contents
Is Air a Mixture or a Pure Substance? A Deep Dive into the Composition of Our Atmosphere
The question of whether air is a mixture or a pure substance is a fundamental one in chemistry and atmospheric science. While it might seem like a simple question with a straightforward answer, a closer examination reveals a fascinating complexity. Understanding the composition of air, its behavior, and the implications of classifying it as a mixture opens a window into the broader world of matter and its properties. This article will delve deeply into this topic, exploring the scientific definitions, evidence supporting air's classification, and the consequences of this classification.
Defining Pure Substances and Mixtures
Before we can definitively classify air, we need to clearly define the terms "pure substance" and "mixture."
Pure Substances: The Building Blocks
A pure substance is a form of matter that has a constant chemical composition and distinct physical properties. This means that no matter where you find a pure substance – whether it's in a lab, a mine, or in nature – it will always have the same properties and be composed of the same elements in the same proportions. Examples of pure substances include elements like oxygen (O₂) and gold (Au), and compounds like water (H₂O) and table salt (NaCl). Pure substances have a definite melting point and boiling point, which are constant under consistent conditions.
Mixtures: A Blend of Components
A mixture, on the other hand, is a combination of two or more substances that are not chemically bonded. Crucially, mixtures can have varying compositions. Think of a salad: the proportions of lettuce, tomatoes, and cucumbers can change from one salad to another. Mixtures can be homogeneous (uniform in composition throughout, like saltwater) or heterogeneous (non-uniform, like a salad). Unlike pure substances, mixtures generally do not have definite melting or boiling points. The properties of a mixture will depend on the properties of its constituent components and their proportions.
The Case for Air as a Mixture
The overwhelming scientific consensus classifies air as a mixture. Several key pieces of evidence support this classification:
Variable Composition: The Defining Characteristic
Perhaps the most compelling evidence is the variable composition of air. The exact proportions of gases in air vary depending on location, altitude, and even time of day. For example:
- Altitude: The concentration of oxygen decreases significantly at higher altitudes. Mountaineers often use supplemental oxygen because the air at high elevations has less oxygen available for respiration.
- Pollution: Urban areas have higher concentrations of pollutants like carbon monoxide (CO) and nitrogen oxides (NOx) compared to rural areas.
- Humidity: The amount of water vapor (H₂O) in the air, which contributes significantly to humidity, fluctuates greatly depending on weather conditions and geographic location.
This variability in composition is a key characteristic of mixtures and directly contradicts the definition of a pure substance, which has a constant composition.
Separation of Components: A Simple Demonstration
Another strong indicator that air is a mixture is the ability to separate its components using physical methods. Liquefaction, distillation, and fractional distillation are common techniques used to separate the gases that make up air. This is done commercially to obtain pure oxygen, nitrogen, and other gases for industrial and medical applications. The fact that we can physically separate the components of air without chemical reactions further supports the classification of air as a mixture. If it were a compound, chemical reactions would be necessary for separation.
Lack of Definite Melting and Boiling Points
Pure substances exhibit sharp melting and boiling points. However, air does not have a specific melting or boiling point. The temperatures at which air transitions between phases depend on the composition of the air sample and the atmospheric pressure. This lack of definite melting and boiling points is another characteristic consistent with air's classification as a mixture.
The Major Components of Air
Let's examine the major components of air to further solidify its classification as a mixture:
- Nitrogen (N₂): Around 78% of dry air is nitrogen. Nitrogen is relatively inert and plays a crucial role in maintaining life as a constituent of proteins and nucleic acids in living organisms.
- Oxygen (O₂): About 21% of dry air is oxygen. Oxygen is essential for respiration in most living organisms and supports combustion.
- Argon (Ar): Argon makes up about 0.93% of dry air. It is an inert noble gas.
- Carbon Dioxide (CO₂): A relatively small but increasingly important component of air, CO₂ levels are rising due to human activities, contributing to climate change.
- Other Gases: Trace amounts of other gases like neon (Ne), helium (He), methane (CH₄), krypton (Kr), hydrogen (H₂), and nitrous oxide (N₂O) are also present in air.
- Water Vapor: The amount of water vapor in air is highly variable, ranging from near zero to several percent.
The presence of these numerous components in varying proportions unequivocally points towards air being a mixture.
The Implications of Air Being a Mixture
The classification of air as a mixture has significant implications across various scientific fields:
Atmospheric Science: Understanding Weather and Climate
Understanding air as a mixture is fundamental to atmospheric science. The varying proportions of gases, particularly water vapor and carbon dioxide, have profound effects on weather patterns and climate. Changes in these proportions can lead to changes in temperature, precipitation, and atmospheric stability.
Environmental Science: Air Pollution and its Effects
The ability to identify and quantify the different components in air is crucial in environmental science, particularly for monitoring and mitigating air pollution. Knowing the specific pollutants present in the air allows scientists to design strategies to reduce their impact on human health and the environment.
Industrial Applications: Gas Separation and Purification
The industrial separation of air's components into pure gases has numerous applications, including the production of oxygen for medical use, nitrogen for food preservation, and argon for welding. These processes rely on the understanding that air is a mixture and its components can be separated physically.
Medical Applications: Respiratory Care and Treatment
The composition of air is of paramount importance in medicine, especially in respiratory care. The precise levels of oxygen and other gases in medical air supplies must be carefully controlled to ensure patient well-being.
Conclusion: Air - A Dynamic Mixture
In conclusion, the scientific evidence overwhelmingly supports the classification of air as a mixture, not a pure substance. Its variable composition, the ability to separate its components using physical methods, the lack of definite melting and boiling points, and the presence of multiple gases in varying proportions all point to this conclusion. Understanding this fundamental classification is essential for advancing our knowledge in atmospheric science, environmental science, industrial applications, and medicine. The ongoing monitoring and analysis of air composition provide crucial insights into the health of our planet and the impact of human activities on our atmosphere. The dynamics of this mixture directly influence our weather, climate, and quality of life, highlighting the importance of continuous research and monitoring of this vital resource.
Latest Posts
Related Post
Thank you for visiting our website which covers about Is Air A Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
