When Gas Turns Into A Liquid
Juapaving
Mar 26, 2025 · 5 min read
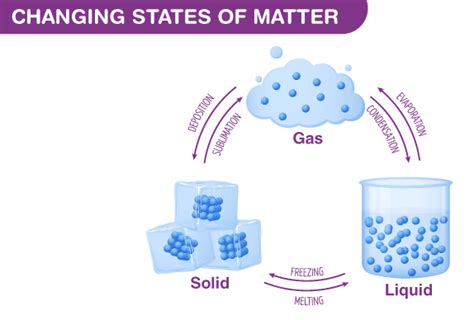
Table of Contents
When Gas Turns Into a Liquid: A Comprehensive Guide to Condensation
The transformation of gas into liquid, a process known as condensation, is a fundamental concept in chemistry and physics. Understanding this phase transition is crucial for various applications, from weather forecasting to industrial processes. This comprehensive guide delves into the intricacies of condensation, exploring the underlying principles, influencing factors, and practical examples of this ubiquitous phenomenon.
Understanding the Basics: Gas vs. Liquid
Before diving into the specifics of condensation, it’s essential to understand the fundamental differences between gases and liquids. Gases are characterized by their lack of definite shape or volume; their particles are widely dispersed and move freely, exhibiting high kinetic energy. Liquids, on the other hand, have a definite volume but no definite shape; their particles are closer together than in gases, exhibiting less kinetic energy and interacting more strongly.
The key difference lies in the intermolecular forces—the attractive forces between molecules. In gases, these forces are weak, allowing molecules to move independently. In liquids, these forces are stronger, holding the molecules closer together, resulting in a more condensed state.
The Condensation Process: From Gas to Liquid
Condensation occurs when a gas transitions into a liquid state. This transition requires a decrease in the kinetic energy of the gas molecules. This reduction in kinetic energy can be achieved through several methods:
1. Cooling: The Most Common Method
The most common way to condense a gas is by lowering its temperature. As the temperature decreases, the gas molecules lose kinetic energy, causing them to move more slowly. This slower movement allows the intermolecular forces to become dominant, pulling the molecules closer together and eventually forming a liquid.
Think about the formation of dew on a cool morning. The water vapor in the air, upon contact with a cooler surface (like grass), loses energy and condenses into tiny water droplets. This is a classic example of condensation driven by cooling.
2. Compression: Increasing Pressure
Another way to induce condensation is by increasing the pressure on the gas. Higher pressure forces the gas molecules closer together, increasing the frequency of intermolecular interactions. This increased interaction leads to a reduction in kinetic energy and facilitates the transition to the liquid phase.
This principle is utilized in various industrial processes, such as liquefying natural gas (LNG) for transportation and storage. By applying high pressure, natural gas, which is typically a gas at ambient conditions, can be condensed into a liquid, significantly reducing its volume and making it easier to handle.
3. Combination of Cooling and Compression
In many real-world scenarios, condensation is achieved by a combination of cooling and compression. This approach optimizes the process, allowing for efficient liquefaction even with gases that are difficult to condense under a single method.
For instance, the liquefaction of air, used in the production of liquid nitrogen and oxygen, relies on a complex system of cooling and compression to achieve the necessary conditions for condensation.
Factors Affecting Condensation
Several factors influence the rate and efficiency of condensation:
1. Temperature: The Driving Force
Temperature is arguably the most significant factor. A lower temperature facilitates condensation by reducing the kinetic energy of gas molecules. The critical temperature, a substance-specific value, represents the temperature above which a gas cannot be liquefied, no matter how high the pressure.
2. Pressure: Proximity and Interaction
Pressure plays a crucial role by influencing the proximity of gas molecules. Higher pressure increases the likelihood of intermolecular interactions, accelerating the condensation process.
3. Surface Area: Sites for Condensation
The surface area available for condensation significantly impacts the rate of the process. A larger surface area provides more sites for gas molecules to adhere and condense, leading to faster condensation. This is why dew forms readily on surfaces with larger areas like leaves and grass.
4. Presence of Condensation Nuclei
The presence of condensation nuclei, microscopic particles such as dust or salt, can significantly enhance condensation. These nuclei provide surfaces for water vapor molecules to condense onto, facilitating the formation of larger droplets. Without these nuclei, condensation may require significantly lower temperatures or higher pressures. This is particularly relevant in cloud formation, where condensation nuclei play a critical role in the formation of raindrops.
5. Type of Gas: Intermolecular Forces
The type of gas influences the condensation process due to differences in intermolecular forces. Gases with stronger intermolecular forces, such as polar molecules, are generally easier to condense than nonpolar gases. This is because stronger forces require less energy reduction to facilitate the transition from a gaseous to a liquid state.
Practical Applications of Condensation
Condensation is a critical process in various applications across multiple fields:
1. Meteorology and Climate: Weather Patterns
Condensation plays a crucial role in meteorological processes. Cloud formation, rain, snow, and fog are all manifestations of condensation. Understanding condensation is paramount for weather forecasting and climate modeling.
2. Industrial Processes: Liquefaction of Gases
The liquefaction of gases, a crucial process in several industries, relies heavily on condensation. Liquefied natural gas (LNG), liquid oxygen (LOX), and liquid nitrogen (LIN) are produced via controlled condensation, facilitating efficient storage and transportation.
3. Refrigeration and Air Conditioning: Cooling Systems
Refrigeration and air conditioning systems rely on the condensation of refrigerants to remove heat from the surrounding environment. The refrigerant vapor condenses, releasing heat, and then evaporates, absorbing heat, completing the cooling cycle.
4. Water Purification: Desalination
Condensation plays a role in some desalination techniques. The process involves evaporating seawater and then condensing the vapor to collect purified water.
Conclusion: The Significance of Condensation
Condensation, the transformation of gas into liquid, is a fundamental phase transition with far-reaching implications across various scientific disciplines and technological applications. Understanding the factors that influence this process allows for control and manipulation, leading to efficient and effective implementation in various industrial processes and environmental studies. From the formation of rain clouds to the operation of refrigeration systems, condensation plays a critical role in shaping our world. Further research into the intricacies of condensation will undoubtedly lead to further advancements and innovation across many fields. The more we understand about this fundamental process, the better equipped we will be to harness its power for the benefit of humanity. From optimizing industrial processes to mitigating climate change, the ongoing study of condensation remains a vital area of scientific inquiry.
Latest Posts
Latest Posts
-
In Which Situation Is The Distance Traveled Proportional To Time
Mar 28, 2025
-
Which Group Of Nonmetals Is The Most Reactive
Mar 28, 2025
-
99 Rounded To The Nearest Tenth
Mar 28, 2025
-
What Percent Is Equivalent To 3 8
Mar 28, 2025
-
What Is The Greatest Common Factor Of 75
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about When Gas Turns Into A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
