Ionic Compounds Are Made Up Of
Juapaving
Mar 13, 2025 · 6 min read
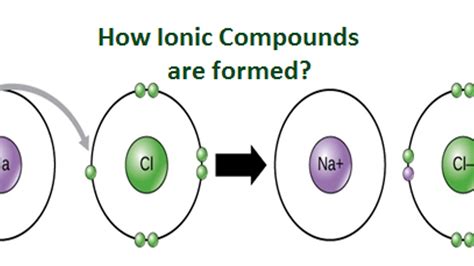
Table of Contents
Ionic Compounds: A Deep Dive into Their Composition and Properties
Ionic compounds are fundamental building blocks of chemistry, forming the basis of numerous materials with diverse applications. Understanding their composition is crucial for grasping their unique properties and behavior. This comprehensive guide delves into the intricate details of ionic compound formation, exploring the constituent ions, their bonding characteristics, and the resulting crystal structures. We'll also examine how these structural features influence the macroscopic properties of ionic compounds.
What are Ionic Compounds Made Of?
At their core, ionic compounds are composed of ions. These ions are atoms or molecules that carry a net electric charge due to a gain or loss of electrons. The process of forming ions is called ionization. There are two main types of ions:
1. Cations: Positively Charged Ions
Cations are formed when an atom loses one or more electrons, resulting in a positive charge. This typically occurs with metals, which have relatively low ionization energies. The number of electrons lost determines the charge of the cation. For example:
- Sodium (Na) loses one electron to form a +1 cation (Na⁺).
- Magnesium (Mg) loses two electrons to form a +2 cation (Mg²⁺).
- Aluminum (Al) loses three electrons to form a +3 cation (Al³⁺).
The tendency of an element to form a cation is directly related to its position in the periodic table. Elements on the left side (alkali and alkaline earth metals) readily form cations.
2. Anions: Negatively Charged Ions
Anions are formed when an atom gains one or more electrons, resulting in a negative charge. This commonly happens with nonmetals, which have a high electron affinity. Again, the number of electrons gained determines the charge of the anion. For instance:
- Chlorine (Cl) gains one electron to form a -1 anion (Cl⁻).
- Oxygen (O) gains two electrons to form a -2 anion (O²⁻).
- Nitrogen (N) gains three electrons to form a -3 anion (N³⁻).
Nonmetals located on the right side of the periodic table (halogens, chalcogens) have a strong tendency to form anions.
The Electrostatic Attraction: The Essence of Ionic Bonding
The fundamental force holding ionic compounds together is electrostatic attraction between the oppositely charged cations and anions. This attractive force is significantly strong, leading to the formation of stable, crystalline structures. This attractive force is described by Coulomb's Law, which states that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
The strength of the ionic bond is influenced by several factors:
- Charge magnitude: Higher charges on the ions lead to stronger attraction. For example, the bond between Mg²⁺ and O²⁻ is stronger than the bond between Na⁺ and Cl⁻.
- Ionic radii: Smaller ions result in stronger attraction because the distance between the charges is reduced.
- Lattice energy: This represents the energy released when gaseous ions combine to form a solid ionic crystal. A higher lattice energy indicates a stronger ionic bond.
Crystal Structure: The Ordered Arrangement of Ions
Ionic compounds do not exist as individual molecules but rather as crystalline solids. This means the ions are arranged in a highly ordered, three-dimensional repeating pattern called a crystal lattice. The specific arrangement depends on the size and charge of the ions involved. Common crystal structures include:
- Simple cubic: A relatively simple arrangement, less common due to its inefficiency in packing ions.
- Body-centered cubic: Ions are located at the corners and the center of the cube.
- Face-centered cubic: Ions are located at the corners and the center of each face of the cube.
- Hexagonal close-packed: A highly efficient arrangement with ions packed closely together.
The crystal structure plays a critical role in determining the physical properties of the ionic compound, such as hardness, melting point, and cleavage.
Properties of Ionic Compounds: A Reflection of their Composition
The unique arrangement and bonding in ionic compounds impart several distinctive properties:
1. High Melting and Boiling Points:
The strong electrostatic forces between ions require a substantial amount of energy to overcome, leading to high melting and boiling points. This is why many ionic compounds are solids at room temperature.
2. Brittleness:
Ionic crystals are brittle because the displacement of layers of ions can cause repulsion between like charges, leading to fracture.
3. Solubility in Polar Solvents:
Ionic compounds often dissolve readily in polar solvents like water. The polar water molecules can interact with and surround the ions, weakening the electrostatic attractions and pulling the ions into solution. This process is called hydration.
4. Conductivity in Molten or Aqueous State:
Ionic compounds are generally poor conductors of electricity in the solid state because the ions are fixed in the crystal lattice. However, when molten or dissolved in water, the ions become mobile and can conduct electricity.
5. Crystalline Structure:
As mentioned earlier, their regular, repeating arrangement of ions creates a crystalline structure which is often visible to the naked eye.
Examples of Ionic Compounds and Their Applications
Numerous ionic compounds are crucial in various applications, highlighting their importance in everyday life and industry:
- Sodium chloride (NaCl): Table salt, essential for human health and used in food preservation and industrial processes.
- Calcium carbonate (CaCO₃): A major component of limestone, marble, and chalk; used in construction materials, antacids, and as a dietary supplement.
- Potassium nitrate (KNO₃): Used in fertilizers, gunpowder, and food preservatives.
- Magnesium oxide (MgO): Used in refractories (materials resistant to high temperatures), in agriculture, and as a dietary supplement.
- Silver chloride (AgCl): Used in photographic film and as an antiseptic.
Beyond Simple Binary Ionic Compounds
While the discussion has focused primarily on binary ionic compounds (compounds formed from two elements), many ionic compounds involve more than two elements and complex polyatomic ions. Polyatomic ions are groups of atoms covalently bonded together that carry a net charge, such as:
- Nitrate (NO₃⁻): Found in fertilizers and explosives.
- Sulfate (SO₄²⁻): Present in many minerals and used in various industrial applications.
- Phosphate (PO₄³⁻): Essential for life, found in DNA, RNA, and fertilizers.
- Hydroxide (OH⁻): A key component in many bases and alkaline solutions.
- Ammonium (NH₄⁺): A cation found in ammonium salts, important in fertilizers and some cleaning products.
Understanding these polyatomic ions is crucial for comprehending the composition and properties of a wider range of ionic compounds. The principles of electrostatic attraction and crystal lattice formation remain central to their behavior.
Conclusion: The Significance of Ionic Compounds
Ionic compounds, formed through the electrostatic attraction of cations and anions, play a significant role in various aspects of our lives. Their unique properties, stemming from their composition and crystal structure, make them essential components in diverse applications, from everyday materials to advanced technologies. A thorough understanding of ionic compounds is fundamental to advancements in materials science, chemistry, and related fields. This deep dive into their composition serves as a solid foundation for further exploration of their fascinating properties and applications. Continued research will undoubtedly uncover even more about the intricacies of ionic compounds and their potential in shaping future technologies.
Latest Posts
Latest Posts
-
Examples Of Right Angles In Real Life
Mar 13, 2025
-
What Is The Charge For Tin
Mar 13, 2025
-
How Much Atp Does The Electron Transport Chain Produce
Mar 13, 2025
-
Are All Rational Numbers Integers True Or False
Mar 13, 2025
-
2 Out Of 8 As A Percentage
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Ionic Compounds Are Made Up Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
