How Much Atp Does The Electron Transport Chain Produce
Juapaving
Mar 13, 2025 · 6 min read
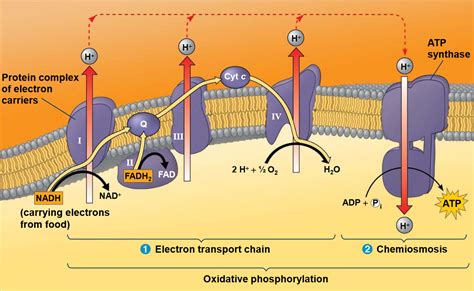
Table of Contents
How Much ATP Does the Electron Transport Chain Produce? A Deep Dive into Oxidative Phosphorylation
The electron transport chain (ETC), also known as the respiratory chain, is the final stage of cellular respiration. It's a crucial process that harvests the energy stored in reduced electron carriers, NADH and FADH₂, to generate a substantial amount of ATP – the cell's primary energy currency. But precisely how much ATP does the ETC produce? The answer, while seemingly simple, requires a nuanced understanding of the complex interplay of protons, electrons, and the electrochemical gradient. This article delves deep into the intricacies of oxidative phosphorylation, exploring the factors influencing ATP yield and clarifying common misconceptions.
Understanding the Electron Transport Chain: A Molecular Machine
The ETC isn't a single entity but a series of protein complexes embedded within the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). These complexes act as a molecular assembly line, sequentially transferring electrons from NADH and FADH₂ to oxygen (the final electron acceptor). This electron transfer is coupled to the pumping of protons (H⁺) across the inner mitochondrial membrane, establishing a proton gradient.
The Key Players: Complexes I-IV and ATP Synthase
- Complex I (NADH dehydrogenase): Accepts electrons from NADH and transfers them to ubiquinone (Q), pumping protons in the process.
- Complex II (succinate dehydrogenase): Enters the ETC at a lower energy level, accepting electrons from FADH₂ and transferring them to ubiquinone without directly pumping protons.
- Complex III (cytochrome bc₁ complex): Receives electrons from ubiquinone and passes them to cytochrome c, further contributing to proton pumping.
- Complex IV (cytochrome c oxidase): The final electron acceptor complex, transferring electrons from cytochrome c to molecular oxygen (O₂), forming water. This complex also pumps protons.
- ATP Synthase: This remarkable enzyme uses the energy stored in the proton gradient (proton-motive force) to synthesize ATP from ADP and inorganic phosphate (Pi). This process is called chemiosmosis.
The ATP Yield: A Variable Equation
The widely cited number for ATP produced per NADH is 2.5, and 1.5 for FADH₂. However, this is a simplification. The actual ATP yield is more complex and influenced by several factors:
- The Proton Motive Force (PMF): The PMF is the driving force behind ATP synthesis. It's determined by both the proton gradient (ΔpH) and the membrane potential (ΔΨ). Variations in the PMF due to factors like temperature and metabolic state affect ATP production.
- The P/O Ratio: This represents the number of ATP molecules synthesized per atom of oxygen reduced. The theoretical P/O ratio for NADH is 3 and for FADH₂ is 2. However, the actual P/O ratio is often less than this ideal due to proton leakage and other inefficiencies.
- Shuttle Systems: NADH generated in glycolysis cannot directly cross the mitochondrial membrane. Different shuttle systems (e.g., the glycerol-3-phosphate shuttle and the malate-aspartate shuttle) transport reducing equivalents into the mitochondria, impacting the number of ATP molecules produced per NADH. The malate-aspartate shuttle yields a higher ATP count than the glycerol-3-phosphate shuttle.
- Substrate-Level Phosphorylation: While the ETC's primary role is oxidative phosphorylation, glycolysis and the citric acid cycle also produce ATP through substrate-level phosphorylation. This contributes to the overall ATP yield of cellular respiration but is independent of the ETC.
Calculating the ATP Yield: A Step-by-Step Approach
Let's consider the complete breakdown of glucose (C₆H₁₂O₆) via cellular respiration to illustrate the ATP yield:
-
Glycolysis: Produces 2 ATP (substrate-level phosphorylation) and 2 NADH.
-
Pyruvate Oxidation: Each pyruvate molecule (2 total from glycolysis) produces 1 NADH. This yields a total of 2 NADH.
-
Citric Acid Cycle (Krebs Cycle): Each acetyl-CoA (from pyruvate oxidation) produces 3 NADH, 1 FADH₂, and 1 ATP (substrate-level phosphorylation). Since there are 2 acetyl-CoA molecules per glucose, this stage produces 6 NADH, 2 FADH₂, and 2 ATP.
-
Electron Transport Chain and Oxidative Phosphorylation:
-
Using the "standard" estimates (2.5 ATP/NADH and 1.5 ATP/FADH₂):
- From glycolysis: 2 NADH * 2.5 ATP/NADH = 5 ATP
- From pyruvate oxidation: 2 NADH * 2.5 ATP/NADH = 5 ATP
- From the citric acid cycle: 6 NADH * 2.5 ATP/NADH = 15 ATP; 2 FADH₂ * 1.5 ATP/FADH₂ = 3 ATP
- Total ATP from ETC: 5 + 5 + 15 + 3 = 28 ATP
-
Total ATP from Cellular Respiration (using standard estimates): 2 (glycolysis) + 2 (citric acid cycle) + 28 (ETC) = 32 ATP
-
Important Note: The actual ATP yield can vary depending on the shuttle system used and the efficiency of the ETC. Some sources cite a total ATP yield closer to 30 or even slightly less.
Factors Affecting ATP Production Efficiency
Several internal and external factors can modulate the efficiency of ATP production by the ETC:
- Temperature: Extreme temperatures can denature proteins, disrupting the ETC's function and reducing ATP synthesis.
- Oxygen Availability: Oxygen is the final electron acceptor. Its absence (anaerobic conditions) halts the ETC, forcing the cell to rely on less efficient anaerobic pathways.
- Inhibitors and Uncouplers: Certain molecules can interfere with the ETC. Inhibitors block electron transport, while uncouplers disrupt the proton gradient, reducing ATP synthesis.
- Metabolic State of the Cell: The energy demands of the cell influence the rate of oxidative phosphorylation. High energy demand leads to increased ATP production.
- Genetic Mutations: Mutations affecting the proteins of the ETC can significantly impact its efficiency and ATP yield.
The Significance of the Electron Transport Chain
The ETC's role in ATP production is paramount to life. The vast majority of ATP generated in aerobic organisms comes from this process. Its efficiency allows organisms to harness a significant amount of energy from the oxidation of glucose and other fuels. Dysfunction in the ETC is implicated in various diseases, highlighting its crucial role in cellular health and metabolism. A deeper understanding of the ETC's complexities allows us to appreciate its intricate machinery and its vital contribution to energy homeostasis within cells.
Conclusion: More Than Just a Number
While a simple answer of "around 32 ATP" is often given for glucose oxidation, the reality is far more intricate. The number of ATP molecules produced by the ETC is not a fixed value but rather a dynamic output influenced by multiple factors. This article aimed to provide a more nuanced understanding of the process, highlighting the complexities and variations that influence the final ATP yield. By understanding these intricacies, we can better appreciate the elegance and importance of this central metabolic pathway. Further research continues to refine our understanding of oxidative phosphorylation, uncovering more details about its regulation and potential for therapeutic interventions.
Latest Posts
Latest Posts
-
Nucleotides Contain A Sugar A Phosphate And A Nitrogenous
May 09, 2025
-
What Are The Two Types Of Mechanical Energy
May 09, 2025
-
Sample Letter Of Refund Payment To Customer
May 09, 2025
-
What Is The Source Of Oxygen Released During Photosynthesis
May 09, 2025
-
How Many Centimeters Is 13 Inches
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Much Atp Does The Electron Transport Chain Produce . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.