If Temperature Increases What Happens To Volume
Juapaving
Mar 25, 2025 · 6 min read
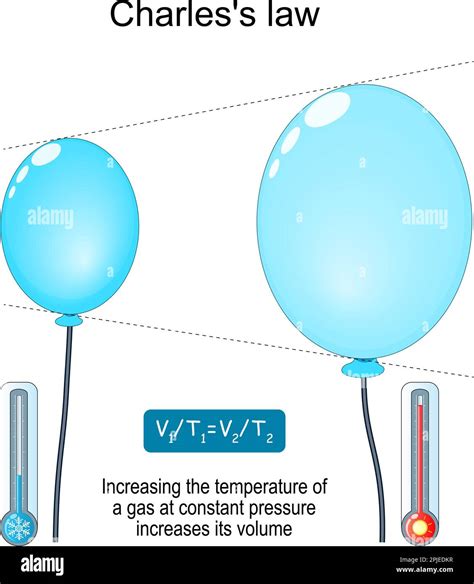
Table of Contents
If Temperature Increases, What Happens to Volume? Exploring Thermal Expansion
The relationship between temperature and volume is a fundamental concept in physics, with far-reaching implications across various fields. Understanding how temperature changes affect volume is crucial in numerous applications, from engineering and construction to meteorology and material science. This article delves into the intricacies of thermal expansion, explaining the underlying principles, exploring different types of expansion, and examining its real-world consequences.
The Basics: Thermal Expansion and its Causes
When the temperature of a substance increases, its particles gain kinetic energy, moving faster and farther apart. This increased particle motion leads to an expansion of the substance's volume. This phenomenon is known as thermal expansion. The degree to which a substance expands is dependent on several factors, including:
-
Material properties: Different materials have different coefficients of thermal expansion. Metals generally expand more than ceramics or polymers. The specific atomic structure and bonding within a material dictate its responsiveness to temperature changes. Understanding the coefficient of thermal expansion for a specific material is critical for accurate predictions of volume changes.
-
Temperature change: The magnitude of the volume change is directly proportional to the temperature change. A larger temperature increase results in a larger volume increase. This relationship is expressed mathematically, as we'll explore later.
-
Initial volume: The larger the initial volume of a substance, the greater the increase in volume for a given temperature change. This seems intuitive: a larger object will naturally expand more than a smaller one under the same temperature conditions.
-
Pressure: While often overlooked, pressure also influences thermal expansion. At higher pressures, the particles are closer together, reducing the extent of expansion. This effect is often negligible at atmospheric pressures but becomes significant in high-pressure applications.
Types of Thermal Expansion: Linear, Area, and Volumetric
Thermal expansion isn't a uniform phenomenon; it manifests differently depending on the dimensions considered:
-
Linear Expansion: This refers to the change in length of a solid object as its temperature changes. It's relevant for long, slender objects like rods or wires. The formula for linear expansion is:
ΔL = αL₀ΔTwhere:
ΔLis the change in lengthαis the coefficient of linear thermal expansion (specific to the material)L₀is the original lengthΔTis the change in temperature
-
Area Expansion: This considers the change in the surface area of an object due to temperature change. The formula for area expansion is:
ΔA = 2αA₀ΔTNote the factor of 2, reflecting the two-dimensional nature of area.
-
Volumetric Expansion: This describes the change in the overall volume of an object, encompassing all three dimensions. This is the most commonly used and relevant form when discussing the relationship between temperature and volume. The formula for volumetric expansion is:
ΔV = βV₀ΔTwhere:
ΔVis the change in volumeβis the coefficient of volumetric thermal expansion (often approximately 3α for isotropic materials)V₀is the original volumeΔTis the change in temperature
Isotropic materials expand uniformly in all directions. However, anisotropic materials expand differently along different axes, requiring more complex calculations to account for their unique expansion properties.
Real-World Applications and Consequences of Thermal Expansion
The effects of thermal expansion are ubiquitous and have significant implications in various engineering and scientific fields:
-
Civil Engineering: Bridges, roads, and buildings are designed to account for thermal expansion. Expansion joints are incorporated into structures to accommodate changes in length due to temperature fluctuations, preventing cracking and damage. Failure to consider thermal expansion can lead to structural failure.
-
Mechanical Engineering: In machinery and engines, thermal expansion plays a crucial role. Precise tolerances must be considered to ensure proper functioning across varying temperatures. For example, the expansion of metal parts in an engine is a critical factor influencing its performance and longevity. Understanding thermal expansion is crucial in designing reliable and efficient machinery.
-
Aerospace Engineering: In aircraft and spacecraft design, thermal expansion significantly impacts the structural integrity and aerodynamic performance. Materials with low coefficients of thermal expansion are preferred in applications where extreme temperature variations are expected.
-
Material Science: The study of thermal expansion provides valuable insights into the atomic structure and bonding within materials. Researchers use thermal expansion data to characterize and classify materials, developing new materials with tailored properties.
-
Thermometry: The principle of thermal expansion is the basis for many liquid-in-glass thermometers. As temperature changes, the liquid expands or contracts, indicating the temperature.
-
Metrology: Accurate measurements require accounting for the effects of thermal expansion on measuring instruments. Temperature-controlled environments are often necessary to minimize measurement errors.
-
Geology: Thermal expansion contributes to rock fracturing and weathering processes, influencing geological formations and landscape evolution. The expansion and contraction of rocks due to temperature fluctuations can create stresses that lead to fracturing and erosion.
Anomalies and Exceptions: Water's Unique Behavior
Water exhibits a unique and anomalous behavior regarding thermal expansion. Between 0°C and 4°C, water actually contracts as its temperature increases. Above 4°C, it expands as expected. This unusual behavior is due to the hydrogen bonding structure of water molecules. This anomaly has crucial implications for aquatic life, preventing lakes and rivers from freezing solid from the bottom up.
Calculating Volume Changes: Examples and Practical Considerations
Let's illustrate the principles of volumetric expansion with a couple of examples:
Example 1: A glass container has a volume of 1000 cm³ at 20°C. The coefficient of volumetric thermal expansion for glass is approximately 27 x 10⁻⁶ °C⁻¹. What will be the volume of the container at 50°C?
Using the formula ΔV = βV₀ΔT, we have:
ΔV = (27 x 10⁻⁶ °C⁻¹)(1000 cm³)(50°C - 20°C) = 0.81 cm³
Therefore, the new volume will be approximately 1000.81 cm³.
Example 2: A metal sphere has a volume of 50 cm³ at 25°C. Its coefficient of volumetric thermal expansion is 36 x 10⁻⁶ °C⁻¹. If the temperature increases to 75°C, what will be the increase in volume?
ΔV = (36 x 10⁻⁶ °C⁻¹)(50 cm³)(75°C - 25°C) = 0.09 cm³
The volume increase is 0.09 cm³.
Important Considerations:
-
Accuracy of Coefficients: The coefficients of thermal expansion are approximate values. Their precise values depend on factors like the purity and composition of the material.
-
Nonlinearity at High Temperatures: The formulas presented assume a linear relationship between temperature and volume change. At very high temperatures, this linearity may break down, requiring more complex models.
-
Phase Transitions: The formulas do not apply during phase transitions (e.g., melting or boiling). Phase transitions involve significant volume changes unrelated to the simple thermal expansion discussed here.
Conclusion: The Significance of Understanding Thermal Expansion
Understanding the relationship between temperature and volume is essential across numerous scientific and engineering disciplines. From designing robust structures to developing new materials and understanding fundamental physical phenomena, the implications of thermal expansion are widespread and significant. This comprehensive overview highlights the fundamental principles, practical applications, and limitations of thermal expansion, equipping readers with a thorough understanding of this critical concept. By considering the effects of thermal expansion, engineers and scientists can design more reliable, efficient, and durable systems, contributing to advancements in various technological fields. The continued study and precise modeling of thermal expansion remain crucial for future technological developments and scientific progress.
Latest Posts
Latest Posts
-
The Minimum Wage Is An Example Of A
Mar 26, 2025
-
Word That Starts With A V
Mar 26, 2025
-
The Red Data Book Keeps A Record Of All The
Mar 26, 2025
-
What Are The Inner Transition Metals
Mar 26, 2025
-
How Many Liters Is 6 Gallons
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about If Temperature Increases What Happens To Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
