How To Find The Mole Of A Compound
Juapaving
Mar 06, 2025 · 5 min read
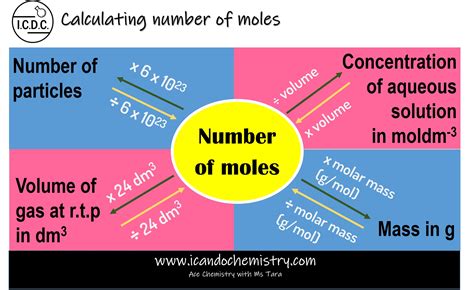
Table of Contents
How to Find the Mole of a Compound: A Comprehensive Guide
Determining the mole of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. Understanding how to accurately calculate moles is essential for anyone studying chemistry, from high school students to advanced researchers. This comprehensive guide will walk you through the process, covering different scenarios and providing practical examples.
Understanding the Mole Concept
Before diving into calculations, let's solidify our understanding of what a mole represents. A mole (mol) is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of entities. These entities can be atoms, molecules, ions, or any other specified particles. Think of it like a dozen (12) – a dozen eggs is 12 eggs, and a mole of atoms is 6.022 x 10²³ atoms. The mole provides a convenient way to relate macroscopic measurements (like grams) to the microscopic world of atoms and molecules.
Key Concepts and Formulas
Several key concepts and formulas are essential for calculating moles:
-
Molar Mass (M): The mass of one mole of a substance, expressed in grams per mole (g/mol). This is numerically equivalent to the atomic weight (for elements) or the sum of atomic weights (for compounds) found on the periodic table.
-
Number of Moles (n): The amount of substance, measured in moles (mol).
-
Mass (m): The amount of substance, measured in grams (g).
-
Avogadro's Number (Nₐ): 6.022 x 10²³ entities/mol.
The most fundamental formula for calculating moles is:
n = m / M
Where:
- n = number of moles
- m = mass in grams
- M = molar mass in g/mol
This formula allows us to calculate the number of moles if we know the mass and molar mass of the substance.
Calculating Moles: Step-by-Step Guide
Let's break down the process of finding the mole of a compound into clear, actionable steps:
Step 1: Identify the Compound and Determine its Chemical Formula
This seemingly simple step is crucial. Knowing the chemical formula allows you to accurately calculate the molar mass. For example, if you're working with water (H₂O), you know the formula and can proceed to the next step.
Step 2: Determine the Molar Mass (M)
This involves using the periodic table to find the atomic mass of each element in the compound. Then, multiply the atomic mass of each element by the number of atoms of that element in the chemical formula and add the results together.
Example: Let's calculate the molar mass of water (H₂O).
- Atomic mass of Hydrogen (H): 1.008 g/mol
- Atomic mass of Oxygen (O): 16.00 g/mol
Molar mass of H₂O = (2 x 1.008 g/mol) + (1 x 16.00 g/mol) = 18.016 g/mol
Step 3: Determine the Mass (m) of the Compound
This is the mass of the compound you are working with, usually given in the problem or measured experimentally. The units must be in grams.
Step 4: Apply the Formula n = m / M
Now, simply substitute the values you found in steps 2 and 3 into the formula:
n = m / M
Example: Let's say you have 10 grams of water (H₂O).
- m = 10 g
- M = 18.016 g/mol (calculated in step 2)
n = 10 g / 18.016 g/mol ≈ 0.555 moles
Therefore, 10 grams of water contains approximately 0.555 moles of water molecules.
Working with Different Compound Types
The process remains consistent across different compound types, but let's look at a few examples:
Example 1: Ionic Compounds (NaCl - Sodium Chloride)
- Chemical Formula: NaCl
- Molar Mass: Atomic mass of Na (22.99 g/mol) + Atomic mass of Cl (35.45 g/mol) = 58.44 g/mol
- Mass: Let's say we have 5 grams of NaCl.
- Calculation: n = 5 g / 58.44 g/mol ≈ 0.0855 moles
Example 2: Molecular Compounds (CO₂ - Carbon Dioxide)
- Chemical Formula: CO₂
- Molar Mass: Atomic mass of C (12.01 g/mol) + (2 x Atomic mass of O (16.00 g/mol)) = 44.01 g/mol
- Mass: Let's say we have 20 grams of CO₂.
- Calculation: n = 20 g / 44.01 g/mol ≈ 0.454 moles
Example 3: Hydrates (CuSO₄·5H₂O - Copper(II) Sulfate Pentahydrate)
Hydrates contain water molecules within their crystal structure. Calculating the molar mass requires considering the water molecules.
- Chemical Formula: CuSO₄·5H₂O
- Molar Mass: Atomic mass of Cu + Atomic mass of S + (4 x Atomic mass of O) + (5 x (2 x Atomic mass of H + Atomic mass of O)) = 249.70 g/mol
- Mass: Let's say we have 25 grams of CuSO₄·5H₂O.
- Calculation: n = 25 g / 249.70 g/mol ≈ 0.100 moles
Advanced Applications and Considerations
The mole concept is fundamental to various advanced chemical calculations:
-
Stoichiometry: Using balanced chemical equations to determine the quantitative relationships between reactants and products in a chemical reaction. Mole calculations are essential for stoichiometric calculations.
-
Solution Chemistry: Calculating molarity (moles of solute per liter of solution), a crucial concept in understanding the concentration of solutions.
-
Gas Laws: Relating the volume, pressure, temperature, and number of moles of a gas using equations like the Ideal Gas Law (PV = nRT).
-
Titrations: Determining the concentration of an unknown solution by reacting it with a solution of known concentration. Mole calculations are crucial in analyzing titration data.
Troubleshooting Common Mistakes
-
Unit Consistency: Ensure all mass measurements are in grams and molar masses are in g/mol. Inconsistent units will lead to incorrect results.
-
Significant Figures: Pay attention to significant figures throughout your calculations. Your final answer should reflect the appropriate number of significant figures based on the given data.
-
Correct Formula: Double-check the chemical formula of the compound to ensure accurate molar mass calculation.
Conclusion
Calculating the mole of a compound is a cornerstone of chemistry. By understanding the mole concept, mastering the formula (n = m/M), and following the step-by-step guide provided, you can confidently tackle various chemical calculations. Remember to practice regularly and pay close attention to detail, particularly regarding units and significant figures. With consistent practice, you'll become proficient in determining the number of moles in any given compound. This foundational skill will be invaluable throughout your chemical studies and beyond.
Latest Posts
Latest Posts
-
Common Factors Of 4 And 10
Mar 06, 2025
-
What Is The Percent Of 2 6
Mar 06, 2025
-
What Is The Lcm Of 3 8
Mar 06, 2025
-
Is A Sound Wave A Transverse Wave
Mar 06, 2025
-
What Is The Electron Configuration For Magnesium
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Mole Of A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
