How To Find How Many Moles
Juapaving
Mar 16, 2025 · 5 min read
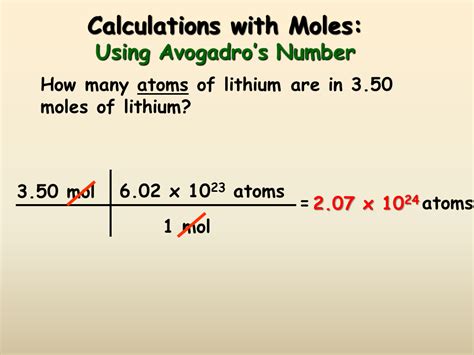
Table of Contents
How to Find the Number of Moles: A Comprehensive Guide
Determining the number of moles is a fundamental concept in chemistry, crucial for various calculations and understanding chemical reactions. This comprehensive guide will explore different methods for finding the number of moles, covering various scenarios and providing practical examples. We'll delve into the core concepts, explore different approaches based on available information, and offer tips for ensuring accuracy in your calculations.
Understanding the Mole Concept
Before diving into the methods, let's establish a clear understanding of what a mole represents. A mole (mol) is a unit of measurement in chemistry that represents a specific number of particles, namely Avogadro's number (6.022 x 10<sup>23</sup>). This number is incredibly large, reflecting the vast number of atoms, molecules, ions, or other elementary entities involved in chemical reactions. Essentially, one mole of any substance contains Avogadro's number of particles.
This concept is vital because it allows us to relate the macroscopic world (grams, liters) to the microscopic world (atoms, molecules). It provides a bridge between the mass of a substance and the number of particles it contains.
Methods for Determining the Number of Moles
The method you use to determine the number of moles depends heavily on the information you have available. Here are the most common methods:
1. Using Mass and Molar Mass
This is arguably the most frequently used method. If you know the mass of a substance and its molar mass, you can calculate the number of moles using the following formula:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
- Mass (g): The mass of the substance in grams. Ensure your mass measurement is accurate.
- Molar Mass (g/mol): The mass of one mole of the substance. This is calculated by summing the atomic masses (found on the periodic table) of all the atoms in the chemical formula.
Example: Calculate the number of moles in 25 grams of water (H₂O).
The molar mass of water is:
- 2 x (atomic mass of Hydrogen) + 1 x (atomic mass of Oxygen) ≈ 2(1.01 g/mol) + 16.00 g/mol ≈ 18.02 g/mol
Therefore:
Moles = 25 g / 18.02 g/mol ≈ 1.39 mol
2. Using Volume and Molar Concentration (Molarity)
This method is commonly used for solutions. If you know the volume of a solution and its molar concentration (molarity), you can calculate the number of moles using:
Moles (mol) = Molarity (mol/L) x Volume (L)
- Molarity (mol/L): The concentration of the solution expressed as moles of solute per liter of solution.
- Volume (L): The volume of the solution in liters. Ensure consistent units (liters).
Example: Calculate the number of moles in 250 mL of a 0.5 M solution of sodium chloride (NaCl).
First, convert the volume to liters: 250 mL = 0.25 L
Then:
Moles = 0.5 mol/L x 0.25 L = 0.125 mol
3. Using Number of Particles and Avogadro's Number
If you know the actual number of particles (atoms, molecules, ions, etc.) present, you can use Avogadro's number to calculate the number of moles:
Moles (mol) = Number of Particles / Avogadro's Number (6.022 x 10<sup>23</sup>)
This method is less frequently used in practical applications but is essential for understanding the fundamental concept of the mole.
Example: Calculate the number of moles in 3.011 x 10<sup>24</sup> molecules of carbon dioxide (CO₂).
Moles = 3.011 x 10<sup>24</sup> / 6.022 x 10<sup>23</sup> ≈ 5 mol
4. Using Gas Laws (Ideal Gas Law)
For gases under ideal conditions, you can use the Ideal Gas Law to calculate the number of moles:
PV = nRT
Where:
- P: Pressure (in atm, Pascals, etc.)
- V: Volume (in L)
- n: Number of moles (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K or other appropriate value depending on the units used)
- T: Temperature (in Kelvin)
This equation requires careful attention to units. Ensure consistency throughout the calculation. The Ideal Gas Law is an approximation; it works best for gases at low pressure and high temperature.
Example: Calculate the number of moles of a gas occupying 5 L at a pressure of 1 atm and a temperature of 298 K. Use R = 0.0821 L·atm/mol·K
n = PV / RT = (1 atm x 5 L) / (0.0821 L·atm/mol·K x 298 K) ≈ 0.204 mol
Advanced Considerations and Potential Challenges
While the above methods provide a solid foundation, several factors can influence the accuracy of your mole calculations:
- Purity of the substance: Impurities in your sample will affect the mass and thus the calculated number of moles.
- Accuracy of measurements: Precise measurements of mass, volume, pressure, and temperature are crucial for obtaining reliable results. Use calibrated instruments whenever possible.
- Deviation from ideal conditions (gases): The Ideal Gas Law assumes ideal conditions. Real gases may deviate from this behaviour, especially at high pressures and low temperatures.
- Stoichiometry: When dealing with chemical reactions, remember to consider the stoichiometric ratios between reactants and products. The coefficients in a balanced chemical equation indicate the mole ratios.
Practical Applications of Mole Calculations
Understanding how to determine the number of moles is crucial in numerous chemical contexts:
- Titrations: Calculating the concentration of a solution using titration data relies on mole calculations.
- Stoichiometry calculations: Predicting the amounts of reactants or products in a chemical reaction requires a thorough understanding of moles and stoichiometric relationships.
- Gas calculations: Many applications involving gases (e.g., combustion, respiration) necessitate the use of the Ideal Gas Law and mole calculations.
- Solution preparation: Accurate preparation of solutions of specific concentrations requires precise mole calculations.
Conclusion
Determining the number of moles is a cornerstone of quantitative chemistry. By mastering the various methods outlined in this guide, including using mass and molar mass, molarity and volume, particle count, and gas laws, you will gain a deeper understanding of chemical quantities and their manipulation. Remember to pay close attention to detail, utilize accurate measurements, and consider potential deviations from ideal conditions to ensure reliable results in your calculations. The ability to accurately determine the number of moles is a critical skill for any chemist, whether working in a laboratory setting or solving theoretical problems. This fundamental concept forms the bedrock of numerous advanced chemical concepts and applications.
Latest Posts
Latest Posts
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
-
Least Common Multiple Of 20 And 3
Mar 17, 2025
-
Function Of The Motor End Plate
Mar 17, 2025
-
A Push Or A Pull Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How To Find How Many Moles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
