How To Determine The Number Of Moles In A Compound
Juapaving
Apr 01, 2025 · 6 min read
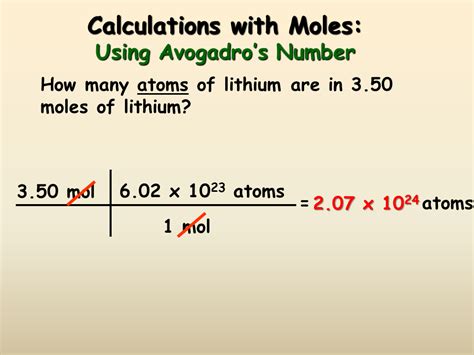
Table of Contents
How to Determine the Number of Moles in a Compound
Determining the number of moles in a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. Understanding this allows you to accurately predict reaction yields, determine concentrations, and perform stoichiometric calculations. This comprehensive guide will walk you through different methods to calculate the number of moles, covering various scenarios and addressing common challenges.
Understanding the Mole Concept
Before delving into the calculations, let's solidify our understanding of the mole. A mole (mol) is a fundamental unit in chemistry representing a specific number of particles, be it atoms, molecules, ions, or formula units. This number, known as Avogadro's number, is approximately 6.022 x 10²³. Essentially, one mole of any substance contains Avogadro's number of particles.
This concept links the macroscopic world (grams, liters) with the microscopic world (atoms, molecules). The beauty lies in its ability to provide a consistent method for comparing and quantifying different substances, regardless of their particle size or mass.
Methods for Determining the Number of Moles
Several methods exist to determine the number of moles in a compound, each tailored to different situations and available information. Let's explore the most common ones:
1. Using Mass and Molar Mass
This is the most frequently used method. It relies on the relationship between the mass of a substance, its molar mass, and the number of moles. The formula is:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
- Mass: This is the weight of the substance, typically measured in grams (g). Accurate measurement is paramount for accurate results. Using a calibrated balance is essential.
- Molar Mass: This is the mass of one mole of a substance. It's calculated by summing the atomic masses of all atoms present in the chemical formula. You can find the atomic mass of each element in the periodic table. For example, the molar mass of water (H₂O) is: (2 x 1.008 g/mol for Hydrogen) + (1 x 16.00 g/mol for Oxygen) = 18.016 g/mol.
Example: What is the number of moles in 10 grams of water (H₂O)?
Moles = 10 g / 18.016 g/mol = 0.555 moles
2. Using Volume and Molar Concentration (Molarity)
This method is particularly useful for solutions. Molarity (M) represents the number of moles of solute per liter of solution. The formula is:
Moles (mol) = Molarity (mol/L) x Volume (L)
- Molarity: This is the concentration of the solution, expressed in moles per liter (mol/L).
- Volume: This is the volume of the solution, expressed in liters (L).
Example: What is the number of moles in 250 mL of a 0.5 M solution of sodium chloride (NaCl)?
First, convert mL to L: 250 mL = 0.250 L
Moles = 0.5 mol/L x 0.250 L = 0.125 moles
3. Using the Number of Particles and Avogadro's Number
This method is less commonly used in practical scenarios but is fundamental for understanding the mole concept. It uses Avogadro's number (6.022 x 10²³) to relate the number of particles to the number of moles:
Moles (mol) = Number of Particles / Avogadro's Number
- Number of Particles: This is the actual count of atoms, molecules, ions, or formula units.
Example: How many moles are present in 3.011 x 10²⁴ molecules of carbon dioxide (CO₂)?
Moles = 3.011 x 10²⁴ / 6.022 x 10²³ = 5 moles
4. Using Gas Laws (Ideal Gas Law)
For gases under ideal conditions, the ideal gas law can be used to determine the number of moles. The formula is:
PV = nRT
Where:
- P: Pressure (in atmospheres, atm)
- V: Volume (in liters, L)
- n: Number of moles (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature (in Kelvin, K)
This method requires knowledge of the gas's pressure, volume, and temperature. Remember that this equation assumes ideal gas behavior, which may not always be accurate under real-world conditions, particularly at high pressures or low temperatures.
Example: A gas occupies 5 L at 25°C (298 K) and 1 atm pressure. How many moles are present?
n = PV/RT = (1 atm * 5 L) / (0.0821 L·atm/mol·K * 298 K) ≈ 0.204 moles
Dealing with Hydrates and Complex Compounds
Calculating moles becomes slightly more complex when dealing with hydrates (compounds containing water molecules) or complex compounds with multiple ions.
Hydrates: Hydrates have water molecules incorporated into their crystal structure. For example, copper(II) sulfate pentahydrate (CuSO₄·5H₂O) contains five water molecules per formula unit. When calculating the molar mass, you must include the mass of the water molecules.
Example: To find the number of moles in 10g of CuSO₄·5H₂O:
-
Calculate the molar mass of CuSO₄·5H₂O: (63.55 + 32.07 + 416.00 + 5(2*1.01 + 16.00)) g/mol ≈ 249.7 g/mol
-
Moles = 10g / 249.7 g/mol ≈ 0.040 moles
Complex Compounds: For ionic compounds, the concept remains the same. Calculate the molar mass by summing the atomic masses of all atoms in the formula unit.
Example: To determine the moles in 5g of Calcium Phosphate, Ca₃(PO₄)₂, you would first calculate the molar mass of Ca₃(PO₄)₂ (3 * atomic mass of Ca + 2 * (atomic mass of P + 4 * atomic mass of O)). Then, divide the given mass by this molar mass to find the number of moles.
Accuracy and Precision: Sources of Error
Several factors can influence the accuracy of mole calculations:
- Measurement errors: Inaccurate weighing or volume measurements directly affect the calculated number of moles.
- Purity of the substance: Impurities in the sample will lead to an inaccurate mass and, therefore, an inaccurate mole calculation.
- Ideal gas law deviations: The ideal gas law assumes ideal gas behavior, which may not always be true, especially at high pressures and low temperatures.
- Stoichiometric ratios: When using mole calculations in stoichiometry, understanding the correct mole ratios from the balanced chemical equation is essential for accurate results.
Applications of Mole Calculations
The ability to determine the number of moles is critical across diverse chemical applications:
- Stoichiometry: Determining reactant and product quantities in chemical reactions.
- Titrations: Calculating the concentration of unknown solutions.
- Solution preparation: Accurately preparing solutions of specific concentrations.
- Gas law calculations: Determining the amount of gas in a given volume under specific conditions.
- Spectroscopy: Relating absorbance or emission to the concentration of a substance.
- Thermochemistry: Calculating heat changes in chemical reactions.
Conclusion
Calculating the number of moles in a compound is a fundamental skill for any chemist or anyone working with chemical quantities. Understanding the various methods—using mass and molar mass, molarity and volume, the number of particles and Avogadro's number, and gas laws—provides the tools to handle diverse scenarios. Always ensure accurate measurements and consider potential sources of error to obtain reliable results. Mastering mole calculations opens the door to a deeper understanding of chemical reactions and processes. Remember to always double-check your calculations and units to avoid mistakes. Practice consistently with various examples to build confidence and proficiency in this essential chemical concept.
Latest Posts
Latest Posts
-
Are All Cells The Same Shape And Size
Apr 02, 2025
-
What Are Rows Called In The Periodic Table
Apr 02, 2025
-
120 Mins Is How Many Hours
Apr 02, 2025
-
How Many Miles Are In 10 Kilometers
Apr 02, 2025
-
Where In A Plant Cell Does Photosynthesis Occur
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Determine The Number Of Moles In A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
