How To Change Mole To Grams
Juapaving
Mar 07, 2025 · 6 min read
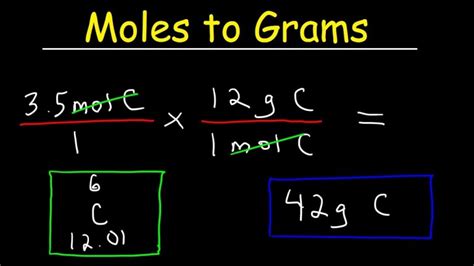
Table of Contents
How to Convert Moles to Grams: A Comprehensive Guide
Converting moles to grams is a fundamental concept in chemistry, crucial for various calculations and experiments. Understanding this conversion is essential for accurately determining the mass of a substance given its molar amount, or vice versa. This comprehensive guide will walk you through the process, providing clear explanations, examples, and troubleshooting tips.
Understanding Moles and Grams
Before diving into the conversion process, let's clarify the concepts of moles and grams.
What is a Mole?
A mole (mol) is a fundamental unit in chemistry representing a specific number of particles, specifically Avogadro's number (6.022 x 10<sup>23</sup>). This number is incredibly large and represents the number of atoms, molecules, ions, or other elementary entities in one mole of a substance. Think of it like a dozen (12), but instead of 12 items, you have 6.022 x 10<sup>23</sup> items. The mole provides a convenient way to handle large numbers of atoms and molecules in chemical reactions.
What is a Gram?
A gram (g) is a unit of mass in the metric system. It measures the amount of matter in a substance. Mass is distinct from weight; mass is a measure of the amount of matter, while weight is a measure of the force of gravity on that matter. In most everyday scenarios, we can treat mass and weight interchangeably, but scientifically, they are different.
The Bridge Between Moles and Grams: Molar Mass
The key to converting between moles and grams lies in understanding molar mass.
Defining Molar Mass
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It essentially tells you how many grams are in one mole of a particular element or compound. For elements, you can find the molar mass on the periodic table; it's numerically equal to the atomic weight. For compounds, you need to calculate the molar mass by summing the molar masses of all the atoms in the chemical formula.
Calculating Molar Mass
Example 1: Finding the molar mass of water (H₂O)
-
Identify the elements and their number: Water (H₂O) contains two hydrogen atoms (H) and one oxygen atom (O).
-
Find the molar mass of each element: From the periodic table:
- Hydrogen (H) has a molar mass of approximately 1.01 g/mol.
- Oxygen (O) has a molar mass of approximately 16.00 g/mol.
-
Calculate the total molar mass:
- (2 x 1.01 g/mol) + (1 x 16.00 g/mol) = 18.02 g/mol
Therefore, the molar mass of water is approximately 18.02 g/mol.
Example 2: Finding the molar mass of sodium chloride (NaCl)
-
Identify the elements and their number: Sodium chloride (NaCl) contains one sodium atom (Na) and one chlorine atom (Cl).
-
Find the molar mass of each element: From the periodic table:
- Sodium (Na) has a molar mass of approximately 22.99 g/mol.
- Chlorine (Cl) has a molar mass of approximately 35.45 g/mol.
-
Calculate the total molar mass:
- (1 x 22.99 g/mol) + (1 x 35.45 g/mol) = 58.44 g/mol
Therefore, the molar mass of sodium chloride is approximately 58.44 g/mol.
Converting Moles to Grams: The Formula
The conversion formula is straightforward:
Grams = Moles x Molar Mass
This formula states that the number of grams of a substance is equal to the number of moles multiplied by the molar mass of that substance.
Worked Examples: Converting Moles to Grams
Let's apply this formula with some examples.
Example 1: Converting 2.5 moles of water to grams
-
Known values:
- Moles = 2.5 mol
- Molar mass of water (H₂O) = 18.02 g/mol (calculated previously)
-
Apply the formula:
- Grams = 2.5 mol x 18.02 g/mol = 45.05 g
Therefore, 2.5 moles of water weighs 45.05 grams.
Example 2: Converting 0.75 moles of carbon dioxide (CO₂) to grams
-
Calculate the molar mass of CO₂:
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol (x2 because there are two oxygen atoms)
- Total molar mass: 12.01 g/mol + (2 x 16.00 g/mol) = 44.01 g/mol
-
Known values:
- Moles = 0.75 mol
- Molar mass of CO₂ = 44.01 g/mol
-
Apply the formula:
- Grams = 0.75 mol x 44.01 g/mol = 33.01 g
Therefore, 0.75 moles of carbon dioxide weighs 33.01 grams.
Example 3: Converting 1.2 moles of glucose (C₆H₁₂O₆) to grams
-
Calculate the molar mass of C₆H₁₂O₆:
- Carbon (C): 12.01 g/mol (x6)
- Hydrogen (H): 1.01 g/mol (x12)
- Oxygen (O): 16.00 g/mol (x6)
- Total molar mass: (6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
-
Known values:
- Moles = 1.2 mol
- Molar mass of C₆H₁₂O₆ = 180.18 g/mol
-
Apply the formula:
- Grams = 1.2 mol x 180.18 g/mol = 216.22 g
Therefore, 1.2 moles of glucose weighs 216.22 grams.
Converting Grams to Moles: The Reverse Calculation
The reverse calculation, converting grams to moles, is equally important. The formula is simply a rearrangement of the previous one:
Moles = Grams / Molar Mass
This means the number of moles is equal to the number of grams divided by the molar mass.
Worked Examples: Converting Grams to Moles
Let's illustrate this with examples.
Example 1: Converting 10 grams of water to moles
-
Known values:
- Grams = 10 g
- Molar mass of water (H₂O) = 18.02 g/mol
-
Apply the formula:
- Moles = 10 g / 18.02 g/mol = 0.555 mol (approximately)
Therefore, 10 grams of water is approximately 0.555 moles.
Example 2: Converting 50 grams of sodium chloride (NaCl) to moles
-
Known values:
- Grams = 50 g
- Molar mass of NaCl = 58.44 g/mol
-
Apply the formula:
- Moles = 50 g / 58.44 g/mol = 0.855 mol (approximately)
Therefore, 50 grams of sodium chloride is approximately 0.855 moles.
Important Considerations and Troubleshooting
-
Significant Figures: Pay close attention to significant figures in your calculations. Your final answer should reflect the precision of your measurements.
-
Unit Consistency: Ensure that your units are consistent throughout the calculation. If you're using grams, make sure your molar mass is also in grams per mole.
-
Accuracy of Molar Mass: The accuracy of your conversion depends on the accuracy of the molar mass used. Using precise molar masses from a reliable source is crucial for accurate results.
-
Complex Compounds: For complex compounds with many atoms, carefully calculate the molar mass to avoid errors. It's helpful to break down the calculation into smaller steps.
-
Hydrates: If you are dealing with hydrates (compounds with water molecules incorporated in their structure), remember to include the mass of the water molecules when calculating the molar mass. For example, the molar mass of copper(II) sulfate pentahydrate (CuSO₄·5H₂O) must include the mass of five water molecules.
Conclusion
Converting moles to grams, and vice versa, is a fundamental skill in chemistry. Mastering this conversion is essential for successful problem-solving in various chemical contexts. By understanding the concepts of moles, grams, and molar mass, and by applying the provided formulas and examples, you can confidently perform these conversions accurately and efficiently. Remember to always double-check your calculations and pay attention to significant figures to ensure the accuracy of your results. Practice makes perfect, so work through several examples to solidify your understanding.
Latest Posts
Latest Posts
-
The Amount Of Matter In An Object Is Called Its
Mar 09, 2025
-
Why Dont Animal Cells Need Chloroplast
Mar 09, 2025
-
Is The Square Root Of 9 A Rational Number
Mar 09, 2025
-
How Many Neutrons Are In Phosphorus
Mar 09, 2025
-
What Is The Percentage Of 3 9
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How To Change Mole To Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
