How Many Neutrons Are In Phosphorus
Juapaving
Mar 09, 2025 · 5 min read
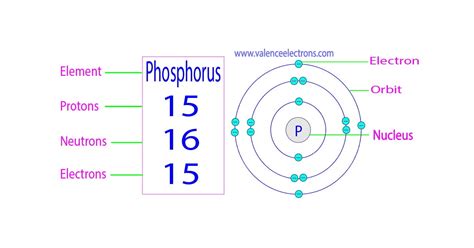
Table of Contents
How Many Neutrons Are in Phosphorus? Understanding Isotopes and Atomic Structure
Determining the number of neutrons in a phosphorus atom isn't as straightforward as simply looking up a single number. The reason lies in the concept of isotopes. This article will delve deep into the atomic structure of phosphorus, explain the significance of isotopes, and ultimately answer the question: how many neutrons are in phosphorus? We'll also explore related concepts like atomic mass, atomic number, and the role of neutrons in nuclear stability.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle the number of neutrons in phosphorus, let's refresh our understanding of atomic structure. An atom is composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all phosphorus atoms have 15 protons. This number is known as the atomic number.
- Neutrons: Neutrally charged particles also found in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
Isotopes: The Key to Variable Neutron Numbers
The key to understanding the variable number of neutrons in phosphorus lies in the concept of isotopes. Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. This means they have the same atomic number but different mass numbers. The mass number is the total number of protons and neutrons in an atom's nucleus.
Phosphorus has several known isotopes, each with a different number of neutrons. The most common isotopes and their characteristics are:
Phosphorus-31 (³¹P) – The Most Abundant Isotope
This is the most prevalent isotope of phosphorus, accounting for nearly 100% of naturally occurring phosphorus. Its atomic number is 15 (15 protons), and its mass number is 31. To find the number of neutrons, we subtract the atomic number from the mass number:
31 (mass number) - 15 (atomic number) = 16 neutrons
Therefore, the most common form of phosphorus, Phosphorus-31, contains 16 neutrons.
Other Phosphorus Isotopes: A Spectrum of Neutron Numbers
While Phosphorus-31 is dominant, other phosphorus isotopes exist, albeit in trace amounts. These isotopes are typically radioactive, meaning their nuclei are unstable and decay over time. Some examples include:
-
Phosphorus-32 (³²P): This isotope has 17 neutrons (32 - 15 = 17). It's a beta emitter, used in various scientific applications, including medical imaging and biological research. Its relatively short half-life (approximately 14.3 days) makes it useful for short-term studies.
-
Phosphorus-33 (³³P): With 18 neutrons (33 - 15 = 18), this isotope is also radioactive, though it has a much longer half-life than Phosphorus-32 (approximately 25.4 days).
-
Other Radioactive Isotopes: Several other radioactive phosphorus isotopes have been identified, each with a different number of neutrons and a corresponding half-life. These isotopes have specialized applications in scientific research.
Atomic Mass and Weighted Average
The atomic mass (or atomic weight) of an element listed on the periodic table is a weighted average of the masses of all its naturally occurring isotopes. This weighted average takes into account the abundance of each isotope. Since Phosphorus-31 is overwhelmingly abundant, the atomic mass of phosphorus is very close to 31 amu (atomic mass units).
The weighted average is crucial because it reflects the typical composition of phosphorus found in nature. While individual phosphorus atoms have a specific number of neutrons (depending on the isotope), the atomic mass gives a representation of the average neutron count across all naturally occurring phosphorus atoms.
The Role of Neutrons in Nuclear Stability
The number of neutrons in an atom's nucleus significantly influences its stability. For lighter elements, a roughly equal number of protons and neutrons generally leads to stable isotopes. However, as the atomic number increases, the ratio of neutrons to protons required for stability increases. This is because the strong nuclear force, which holds the nucleus together, is relatively short-range, while the electrostatic repulsion between positively charged protons acts over a longer distance. Additional neutrons help to overcome this repulsive force and maintain nuclear stability.
In the case of phosphorus, the most stable and abundant isotope, Phosphorus-31, has a neutron-to-proton ratio close to 1:1, contributing to its stability. The radioactive isotopes of phosphorus, with their different neutron-to-proton ratios, have unstable nuclei that decay over time, emitting radiation in the process.
Applications of Phosphorus Isotopes
The different isotopes of phosphorus have diverse applications across various fields:
-
Phosphorus-31 Nuclear Magnetic Resonance (NMR): ³¹P NMR is a powerful technique used in chemistry, biochemistry, and medicine to study the structure and dynamics of molecules containing phosphorus.
-
Phosphorus-32 in Biological Research: ³²P is frequently used as a radioactive tracer in biological experiments. It's incorporated into molecules, allowing researchers to track their movement and fate within living organisms.
-
Phosphorus-32 in Medical Applications: ³²P has had historical uses in cancer treatment, although newer techniques have largely replaced it. Its short half-life is beneficial for localized treatment.
Conclusion: The Number of Neutrons in Phosphorus Depends on the Isotope
To summarize, the simple answer to "How many neutrons are in phosphorus?" is not a single number. The number of neutrons varies depending on the specific isotope. While the most abundant isotope, Phosphorus-31, contains 16 neutrons, other isotopes have different neutron counts, influencing their stability and applications. Understanding the concept of isotopes and the role of neutrons in nuclear stability is crucial to comprehending the diverse properties and applications of phosphorus in various scientific and technological fields. The atomic mass of phosphorus, a weighted average reflecting the natural abundance of its isotopes, provides a broader perspective on the typical number of neutrons found in phosphorus samples encountered in nature.
Latest Posts
Latest Posts
-
What Type Of Symmetry Do Jellyfish Have
Mar 09, 2025
-
Is Dissolving Sugar In Water A Chemical Or Physical Change
Mar 09, 2025
-
Are The Diagonals Of A Trapezoid Congruent
Mar 09, 2025
-
Six Letter Word That Begins With S
Mar 09, 2025
-
What Is 0 5 As A Percent
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
