How Much Atp Does The Etc Produce
Juapaving
Mar 29, 2025 · 5 min read
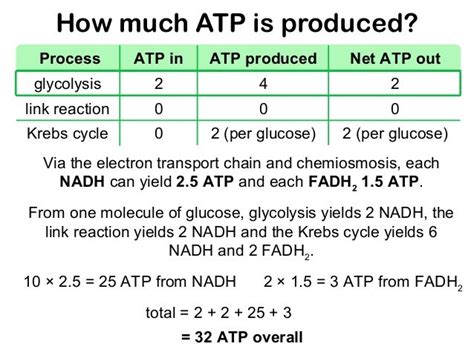
Table of Contents
How Much ATP Does the Electron Transport Chain Produce? A Deep Dive into Oxidative Phosphorylation
The electron transport chain (ETC), also known as the respiratory chain, is a fundamental component of cellular respiration, a process that generates the energy currency of life: ATP (adenosine triphosphate). Understanding exactly how much ATP the ETC produces is crucial for grasping cellular energetics and the overall efficiency of energy metabolism. However, giving a single definitive number is misleading, as the actual ATP yield varies depending on several factors. This article delves into the complexities of oxidative phosphorylation, explaining the process and the factors influencing the final ATP count.
The Electron Transport Chain: A Molecular Powerhouse
The ETC is located within the inner mitochondrial membrane in eukaryotes and the plasma membrane in prokaryotes. It's a series of protein complexes (Complex I-IV) and mobile electron carriers (ubiquinone and cytochrome c) that facilitate the transfer of electrons from electron donors (like NADH and FADH2, generated during glycolysis and the citric acid cycle) to a final electron acceptor, molecular oxygen (O2). This electron flow drives proton (H+) pumping across the inner mitochondrial membrane, establishing a proton gradient.
The Crucial Role of Proton Pumping
The key to ATP production lies in the proton gradient created by the ETC. As electrons move down the chain, energy is released, which is harnessed by Complexes I, III, and IV to pump protons from the mitochondrial matrix to the intermembrane space. This creates a proton motive force (PMF), comprising both a chemical gradient (difference in proton concentration) and an electrical gradient (difference in charge across the membrane).
Chemiosmosis: The Engine of ATP Synthesis
The PMF drives ATP synthesis via a process called chemiosmosis. Protons flow back into the matrix through ATP synthase, a molecular turbine embedded in the inner mitochondrial membrane. The flow of protons through ATP synthase causes a conformational change in the enzyme, driving the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate (Pi). This process is called oxidative phosphorylation because it links the oxidation of electron carriers to the phosphorylation of ADP to ATP.
Calculating ATP Yield: The Complicated Reality
While textbooks often cite a simplified yield of approximately 32 ATP molecules per glucose molecule, this is a highly idealized value. The actual ATP yield is significantly influenced by several factors:
1. The P/O Ratio: A Variable Measure
The phosphorylation-to-oxidation (P/O) ratio represents the number of ATP molecules synthesized per pair of electrons transferred to oxygen. This ratio is not a constant value. While generally accepted values are ~2.5 ATP per NADH and ~1.5 ATP per FADH2, these are estimations and can fluctuate based on the specific conditions within the cell. Factors like the membrane potential, temperature, and the availability of ADP and Pi can subtly affect the efficiency of ATP synthase.
2. Shuttle Systems: NADH's Variable Fate
NADH generated in the cytoplasm during glycolysis doesn't directly enter the mitochondrial matrix. It relies on shuttle systems, which transport reducing equivalents (electrons) across the mitochondrial membrane. The specific shuttle system used (e.g., the glycerol-phosphate shuttle or the malate-aspartate shuttle) impacts the number of ATP molecules ultimately produced per NADH molecule. The malate-aspartate shuttle is more efficient, yielding a higher ATP count per NADH.
3. Proton Leak: Inefficient Energy Expenditure
Protons can leak back across the inner mitochondrial membrane without passing through ATP synthase. This proton leak, or mitochondrial uncoupling, reduces the efficiency of oxidative phosphorylation. While a certain level of proton leak is normal and has regulatory functions, excessive leakage significantly diminishes ATP production. Uncoupling proteins (UCPs) facilitate this proton leak, and their activity can vary depending on factors like temperature and metabolic state.
4. Substrate-Level Phosphorylation: A Non-ETC Contribution
It's crucial to remember that ATP is also produced through substrate-level phosphorylation during glycolysis and the citric acid cycle. These processes occur independently of the ETC, contributing a significant, albeit smaller, portion of the total ATP generated from glucose. This further complicates the calculation of the ETC's specific ATP yield.
Factors Affecting ATP Production: A Deeper Look
Beyond the P/O ratio and shuttle systems, several other factors modulate ATP production:
-
Oxygen Availability: The ETC requires oxygen as the final electron acceptor. In the absence of oxygen (anaerobic conditions), the ETC shuts down, and ATP production drastically reduces. Alternative pathways like fermentation become necessary to generate a small amount of ATP.
-
ADP and Pi Levels: ATP synthesis is dependent on the availability of ADP and inorganic phosphate (Pi). High ATP levels inhibit further ATP synthesis, while low ADP and Pi levels limit the rate of the process.
-
Metabolic State: The energy demands of the cell dictate the rate of oxidative phosphorylation. During periods of high energy demand, the ETC operates at a higher capacity, maximizing ATP production.
-
Hormonal Regulation: Hormones like thyroid hormones influence the rate of metabolism and, consequently, the activity of the ETC.
The Net ATP Yield: An Estimation, Not a Constant
Considering all these variables, a precise number for the ATP yield of the ETC is difficult to provide. While a commonly cited value is around 28-34 ATP per glucose molecule (with the ETC contributing the majority), this is a rough estimation. The actual number will vary depending on the specific cell type, metabolic conditions, and the factors discussed above.
Conclusion: Understanding the Nuances of ATP Production
The electron transport chain plays a pivotal role in cellular energy production, generating the bulk of ATP required by the cell. However, calculating the exact ATP yield from the ETC is complex due to the involvement of several variable factors. Rather than focusing on a single numerical answer, it's more crucial to understand the intricate interplay of different components and their influence on oxidative phosphorylation efficiency. This nuanced understanding is vital for appreciating the dynamic nature of cellular metabolism and its adaptation to diverse physiological conditions. Further research continuously refines our understanding of this critical process, leading to a more comprehensive appreciation of cellular energy production.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 3 And 2
Apr 01, 2025
-
Difference Between Biological Science And Biology
Apr 01, 2025
-
What Is The Least Common Multiple Of 12 And 15
Apr 01, 2025
-
Carbon Dioxide And Water Combine To Form
Apr 01, 2025
-
What Is Lcm Of 3 And 8
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Much Atp Does The Etc Produce . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
