How Many Valence Electrons In S
Juapaving
Mar 26, 2025 · 5 min read
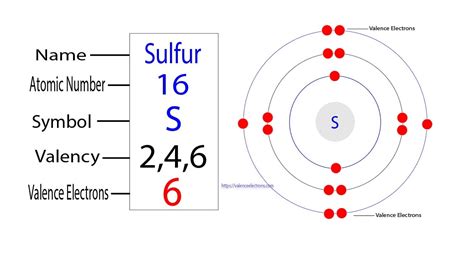
Table of Contents
How Many Valence Electrons in s-Block Elements? A Deep Dive into Group 1 and 2
Understanding valence electrons is fundamental to grasping chemical bonding and reactivity. This comprehensive guide delves into the number of valence electrons found in the s-block elements, specifically focusing on Groups 1 (alkali metals) and 2 (alkaline earth metals) of the periodic table. We'll explore their electronic configurations, explain the significance of valence electrons, and discuss how this impacts their chemical behavior.
What are Valence Electrons?
Valence electrons are the outermost electrons in an atom. They are the electrons most loosely bound to the nucleus and, therefore, most involved in chemical reactions. These electrons determine an element's chemical properties, its ability to form bonds with other atoms, and its overall reactivity. The number of valence electrons largely dictates how many bonds an atom can form and the types of bonds it prefers.
The Significance of the s-Block Elements
The s-block elements, located in Groups 1 and 2 of the periodic table, are characterized by their valence electrons residing primarily in the s subshell. This simple electronic configuration significantly influences their chemical behavior, making them relatively easy to understand and study.
Group 1: Alkali Metals (Li, Na, K, Rb, Cs, Fr)
Alkali metals possess a single valence electron in their outermost s subshell. This lone valence electron is easily lost, resulting in the formation of a +1 cation. This ease of ionization is what accounts for their high reactivity.
Electronic Configurations and Valence Electrons:
- Lithium (Li): 1s²2s¹ (1 valence electron)
- Sodium (Na): 1s²2s²2p⁶3s¹ (1 valence electron)
- Potassium (K): 1s²2s²2p⁶3s²3p⁶4s¹ (1 valence electron)
- Rubidium (Rb): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹ (1 valence electron)
- Caesium (Cs): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s¹ (1 valence electron)
- Francium (Fr): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s¹ (1 valence electron)
Chemical Behavior:
The single valence electron makes alkali metals highly reactive. They readily lose this electron to form a stable +1 ion, readily reacting with water, oxygen, and halogens. Their reactivity increases down the group, with Francium being the most reactive due to its larger atomic size and lower ionization energy.
Group 2: Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra)
Alkaline earth metals have two valence electrons, both residing in their outermost s subshell. These two electrons are more tightly bound than the single electron in alkali metals, resulting in slightly lower reactivity compared to Group 1 elements. However, they still readily lose these two electrons to form a stable +2 cation.
Electronic Configurations and Valence Electrons:
- Beryllium (Be): 1s²2s² (2 valence electrons)
- Magnesium (Mg): 1s²2s²2p⁶3s² (2 valence electrons)
- Calcium (Ca): 1s²2s²2p⁶3s²3p⁶4s² (2 valence electrons)
- Strontium (Sr): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s² (2 valence electrons)
- Barium (Ba): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s² (2 valence electrons)
- Radium (Ra): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s² (2 valence electrons)
Chemical Behavior:
Alkaline earth metals are less reactive than alkali metals but still exhibit significant reactivity. They readily lose their two valence electrons to form stable +2 ions. Their reactivity, like alkali metals, increases down the group due to increased atomic size and decreased ionization energy.
Comparing Group 1 and Group 2 Reactivity
The difference in reactivity between Group 1 and Group 2 is primarily due to the number of valence electrons and the effective nuclear charge. The higher nuclear charge in Group 2 elements makes the valence electrons slightly harder to remove compared to Group 1, leading to lower reactivity.
Beyond the Basics: Orbital Overlap and Bonding
The valence electrons are not just passively present; they actively participate in chemical bonding. Understanding the orbital overlap that occurs during bond formation provides further insight into the chemical behavior of s-block elements.
The single s orbital in Group 1 elements contributes one electron to a covalent bond, creating a single bond. Similarly, the two s orbitals in Group 2 elements contribute two electrons, although often resulting in ionic bonding rather than strictly covalent bonds. The tendency towards ionic bonding is more prominent due to the significant electronegativity difference between s-block metals and many non-metals.
Applications of s-Block Elements
The unique properties of s-block elements translate into a wide range of applications:
- Lithium: Used in batteries (lithium-ion batteries) due to its high electrochemical potential.
- Sodium: Used in sodium vapor lamps for efficient lighting and in sodium chloride (table salt).
- Potassium: Crucial for biological processes, including nerve impulse transmission.
- Magnesium: Utilized in lightweight alloys for aerospace and automotive applications.
- Calcium: Essential for bone health and various biological functions.
Conclusion: Valence Electrons as Key to Understanding Reactivity
The number of valence electrons in s-block elements – one for Group 1 and two for Group 2 – is the key to understanding their chemical behavior. This single or double valence electron is readily lost, leading to the formation of stable cations and resulting in the high reactivity characteristic of these elements. Understanding this fundamental concept provides a solid foundation for appreciating the diverse applications and important roles these elements play in various fields, from technology to biology. Further exploration into the concepts of ionization energy, electron affinity, and electronegativity will provide an even deeper understanding of their chemical properties.
Latest Posts
Latest Posts
-
How Do You Find The Relative Abundance
Mar 26, 2025
-
What Are The Factors For 86
Mar 26, 2025
-
Electric Field Due To Infinite Line Charge
Mar 26, 2025
-
How Many Chromosomes Does Gametes Have
Mar 26, 2025
-
Whats The Greatest Common Factor Of 12 And 18
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In S . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
