How Many Valence Electrons In P
Juapaving
Mar 09, 2025 · 6 min read
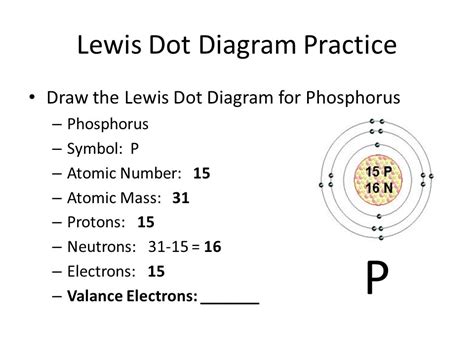
Table of Contents
How Many Valence Electrons in P (Phosphorus)? Understanding Valence Electrons and Their Importance
Phosphorus (P), a crucial element for life, plays a significant role in biological processes and various industrial applications. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article delves deep into the concept of valence electrons, focusing specifically on phosphorus and its implications.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (valence shell) of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of bonds it can form. They are responsible for the chemical properties of an element and how it interacts with other atoms. The number of valence electrons directly influences an element's oxidation state and its ability to gain, lose, or share electrons to achieve a stable electron configuration, often following the octet rule (eight electrons in the valence shell).
Determining Valence Electrons: The Electron Configuration
The number of valence electrons can be determined by examining an element's electron configuration. Electron configuration describes how electrons are distributed among the different energy levels and sublevels within an atom. For phosphorus (P), its atomic number is 15, meaning it has 15 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p³.
Let's break this down:
- 1s²: Two electrons in the first energy level (shell), in the s sublevel.
- 2s²: Two electrons in the second energy level, in the s sublevel.
- 2p⁶: Six electrons in the second energy level, in the p sublevel.
- 3s²: Two electrons in the third energy level, in the s sublevel.
- 3p³: Three electrons in the third energy level, in the p sublevel.
The outermost shell for phosphorus is the third energy level (n=3). This shell contains a total of 5 electrons (2 from the 3s sublevel and 3 from the 3p sublevel). Therefore:
Phosphorus (P) has 5 valence electrons.
The Significance of Phosphorus's 5 Valence Electrons
The presence of five valence electrons significantly influences phosphorus's chemical behavior:
-
Covalent Bonding: Phosphorus readily forms covalent bonds, sharing its valence electrons with other atoms to achieve a more stable electron configuration. This is evident in molecules like phosphine (PH₃) and phosphorus pentachloride (PCl₅). In phosphine, phosphorus shares three electrons to form three single bonds with three hydrogen atoms. In phosphorus pentachloride, phosphorus shares five electrons to form five single bonds with five chlorine atoms. This ability to form multiple bonds is a direct consequence of its five valence electrons.
-
Oxidation States: Due to its five valence electrons, phosphorus can exhibit various oxidation states, ranging from -3 (e.g., in phosphides) to +5 (e.g., in phosphates). This versatility allows phosphorus to participate in a wide range of chemical reactions and form diverse compounds. Its ability to both gain and lose electrons contributes to this versatility. The ability to form different oxidation states is crucial in its biological roles and industrial applications.
-
Allotropes: Phosphorus exists in several allotropic forms, meaning it can exist in different structural modifications with varying properties. White phosphorus, red phosphorus, and black phosphorus are examples. These differences in structure are partly attributed to the ways in which the five valence electrons interact and form bonds.
-
Biological Importance: The 5 valence electrons play a vital role in phosphorus's biological significance. Phosphorus is a crucial component of DNA, RNA, ATP (adenosine triphosphate – the energy currency of cells), and phospholipids (components of cell membranes). The ability to form strong covalent bonds and participate in various chemical reactions, all due to its 5 valence electrons, makes it indispensable for life.
Comparing Phosphorus to Other Group 15 Elements
Phosphorus belongs to Group 15 (also known as Group VA or the pnictogens) in the periodic table. Other elements in this group include nitrogen (N), arsenic (As), antimony (Sb), and bismuth (Bi). They all share a common characteristic: five valence electrons. However, their properties and reactivity differ based on factors like atomic size and electronegativity.
| Element | Atomic Number | Electron Configuration | Valence Electrons |
|---|---|---|---|
| Nitrogen (N) | 7 | 1s²2s²2p³ | 5 |
| Phosphorus (P) | 15 | 1s²2s²2p⁶3s²3p³ | 5 |
| Arsenic (As) | 33 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³ | 5 |
| Antimony (Sb) | 51 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p³ | 5 |
| Bismuth (Bi) | 83 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p³ | 5 |
While all possess five valence electrons, their reactivity decreases as you move down the group. Nitrogen is highly reactive, forming strong triple bonds with itself (N₂) and readily forming covalent bonds with other elements. Phosphorus is less reactive than nitrogen but still readily forms various compounds. As you move down the group to arsenic, antimony, and bismuth, the reactivity further decreases due to increased atomic size and shielding effects.
Applications of Phosphorus and its Compounds
The unique properties arising from its five valence electrons make phosphorus essential in numerous applications:
-
Fertilizers: Phosphate-containing fertilizers are crucial for agriculture, supplying phosphorus, a vital nutrient for plant growth. The high reactivity and ability to form strong bonds are key to its role in plant nutrition.
-
Detergents: Phosphates were historically used in detergents as water softeners, but their use is now restricted due to environmental concerns.
-
Food Additives: Phosphates are used as food additives, acting as emulsifiers, leavening agents, and preservatives. Their ability to interact with various food components makes them useful in food processing.
-
Metallurgy: Phosphorus is used in the production of certain alloys, impacting their properties and performance.
-
Medical Applications: Phosphorus-containing compounds play a role in various medical applications, including medications and imaging agents. The ability to form different bonds and interact with biological molecules is crucial for these applications.
Conclusion: The Importance of Valence Electrons in Understanding Phosphorus
The five valence electrons in phosphorus are fundamental to understanding its chemical behavior, reactivity, and its wide range of applications. From its role in biological systems to its industrial uses, the properties stemming from its electronic structure are crucial. This article provides a comprehensive overview of valence electrons, their significance, and how they specifically relate to the properties and applications of phosphorus. Understanding valence electrons is a cornerstone of chemistry, providing insight into the interactions between atoms and molecules, leading to a deeper comprehension of the material world around us. Further research into the specific applications and compounds of phosphorus can reveal even more about the profound impact of its five valence electrons. The ability to predict and understand chemical behaviour based on the number of valence electrons is a powerful tool in various scientific and engineering disciplines.
Latest Posts
Latest Posts
-
What Are The Factors For 13
Mar 09, 2025
-
How To Get The Diameter Of A Sphere
Mar 09, 2025
-
What Color Is An Animal Cell
Mar 09, 2025
-
What Is The Lcm For 5 And 9
Mar 09, 2025
-
Which Layer Of The Sun Is The Hottest
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In P . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
