How Many Valence Electrons In Iodine
Juapaving
Apr 06, 2025 · 5 min read
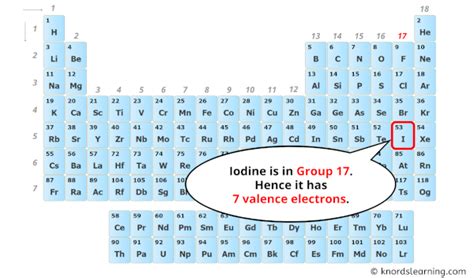
Table of Contents
How Many Valence Electrons Does Iodine Have? A Deep Dive into Iodine's Electronic Structure
Iodine, a fascinating element with a rich history and diverse applications, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to grasping its chemical behavior and reactivity. This comprehensive guide will explore the concept of valence electrons, delve into iodine's electronic configuration, explain why it has the number of valence electrons it does, and examine the implications of this for its chemical properties. We'll also touch upon some interesting applications of iodine leveraging its unique valence electron count.
Understanding Valence Electrons
Before diving into iodine's specifics, let's establish a firm understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (energy level) of an atom. These electrons are the primary players in chemical bonding, determining an atom's reactivity and the types of bonds it can form. They are the electrons most readily involved in interactions with other atoms, forming ionic, covalent, or metallic bonds. The number of valence electrons an atom possesses dictates its position in the periodic table and profoundly influences its chemical properties.
Iodine's Position in the Periodic Table
Iodine (I) is located in Group 17 (also known as Group VIIA or the halogens) of the periodic table. This group is characterized by elements that have seven valence electrons. This consistent feature is a key determinant of their similar chemical behaviors. The elements in this group are highly reactive nonmetals, readily gaining one electron to achieve a stable octet (eight electrons) in their outermost shell, mimicking the electronic configuration of a noble gas.
Determining Iodine's Electronic Configuration
To definitively determine the number of valence electrons in iodine, we need to examine its electronic configuration. This configuration describes how electrons are distributed among the various energy levels and sublevels within the atom. Iodine's atomic number is 53, meaning it has 53 protons and 53 electrons in a neutral atom. Its electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵
Let's break this down:
- Principal Energy Levels (n): The numbers (1, 2, 3, 4, 5) represent the principal energy levels or shells. The higher the number, the farther the electrons are from the nucleus and the higher their energy.
- Sublevels (s, p, d, f): The letters (s, p, d, f) represent sublevels within each principal energy level. Each sublevel can hold a specific number of electrons: s (2), p (6), d (10), f (14).
- Electron Occupancy: The superscripts (², ⁶, ¹⁰, ⁵) indicate the number of electrons occupying each sublevel.
Identifying Iodine's Valence Electrons
To identify iodine's valence electrons, we focus on the highest principal energy level, which, in iodine's case, is the fifth energy level (n=5). This level contains the 5s and 5p sublevels. The 5s sublevel has 2 electrons, and the 5p sublevel has 5 electrons. Therefore, iodine has a total of 2 + 5 = 7 valence electrons.
Why 7 Valence Electrons?
The seven valence electrons in iodine are a direct consequence of its position in the periodic table and its electronic configuration. Its incomplete outermost shell (five electrons in the 5p orbital and two in the 5s orbital) drives its reactivity. To achieve a stable octet, like the noble gases, iodine readily gains one electron, forming an iodide ion (I⁻) with a full octet and a -1 charge. This tendency to gain an electron is characteristic of all halogens, which is directly linked to their seven valence electrons.
Chemical Implications of Iodine's Valence Electrons
The presence of seven valence electrons significantly impacts iodine's chemical behavior:
- Reactivity: Iodine is a reactive nonmetal, readily forming ionic compounds with metals and covalent compounds with nonmetals. Its tendency to gain one electron to complete its octet drives the formation of these bonds.
- Oxidation States: Iodine can exhibit various oxidation states, ranging from -1 (iodide ion) to +7 (in compounds like iodic acid, HIO₃). This variability reflects its ability to either gain or lose electrons in chemical reactions.
- Bond Formation: Iodine forms single covalent bonds in many compounds. This includes simple diatomic iodine (I₂) and a wide range of organic iodine compounds. The single bond reflects the need to share one electron to attain a full octet.
Applications of Iodine Leveraging its Valence Electrons
The unique properties of iodine, stemming from its seven valence electrons, translate to a wide range of applications:
- Medical Applications: Iodine is crucial in the production of thyroid hormones (thyroxine and triiodothyronine), vital for regulating metabolism. Iodine deficiency can lead to goiter and other health problems. Iodine-based disinfectants are also commonly used in healthcare settings.
- Industrial Applications: Iodine compounds find applications in various industries, including photography (silver iodide in photographic film), catalysis (in organic synthesis), and water purification.
- Agricultural Applications: Iodine plays a role in animal nutrition and can be added to animal feed.
Iodine in everyday life:
Beyond the scientific and industrial applications, iodine also affects our daily lives in subtle yet crucial ways:
- Iodized salt: The addition of iodine to table salt is a critical public health measure to combat iodine deficiency.
- Contrast agents in medical imaging: Iodine compounds are commonly used in X-ray and CT scans as contrast agents to enhance the visibility of certain tissues and organs.
Conclusion: The Significance of Iodine's Seven Valence Electrons
In conclusion, iodine possesses seven valence electrons, a defining characteristic that profoundly influences its chemical behavior and reactivity. Its position in Group 17 of the periodic table, coupled with its electronic configuration, explains this crucial aspect of its atomic structure. This number of valence electrons explains its tendency to gain one electron to form a stable octet, leading to its versatility in forming various chemical bonds and its widespread applications in diverse fields from medicine and industry to agriculture and beyond. Understanding the number of valence electrons in an element is a fundamental step in comprehending its chemical properties and its role in the world around us, and for iodine, these seven valence electrons are the key to unlocking its rich chemical story.
Latest Posts
Latest Posts
-
Is Potassium Oxide Ionic Or Covalent
Apr 07, 2025
-
6 Quarts Is How Many Cups
Apr 07, 2025
-
Difference Between A Variable And A Constant
Apr 07, 2025
-
5 Letter Words Starting With Po
Apr 07, 2025
-
The Sum Of 5 Consecutive Odd Numbers Of 135
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Iodine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
