How Many Valence Electrons Does Group 16 Have
Juapaving
Mar 16, 2025 · 5 min read
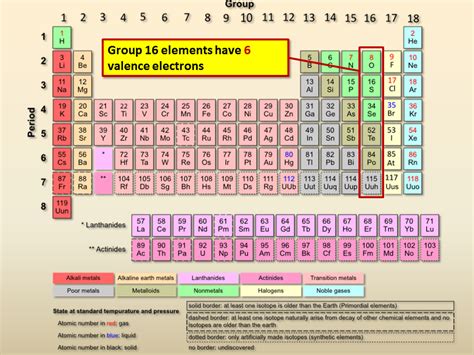
Table of Contents
How Many Valence Electrons Does Group 16 Have? A Deep Dive into the Chemistry of Chalcogens
The question, "How many valence electrons does Group 16 have?" might seem simple at first glance. The answer, however, opens the door to a fascinating exploration of chemical bonding, periodic trends, and the unique properties of this group of elements, also known as the chalcogens. This article will delve into the intricacies of Group 16, explaining not just the number of valence electrons but also how this number dictates their reactivity, bonding patterns, and the diverse roles they play in the natural world and various applications.
Understanding Valence Electrons: The Key to Reactivity
Before we dive into the specifics of Group 16, let's establish a firm understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they are the ones involved in chemical bonding with other atoms. The number of valence electrons determines an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). Atoms strive for stability, often achieving this by gaining, losing, or sharing valence electrons to obtain a full outermost shell, usually eight electrons (octet rule). Exceptions exist, especially with elements having low atomic numbers.
Group 16: The Chalcogens – A Family of Diverse Elements
Group 16, also known as the oxygen group or chalcogens, is a diverse collection of elements that share a common characteristic: they all have six valence electrons. This fundamental similarity leads to some shared properties, although significant differences emerge due to variations in atomic size and electronegativity as you move down the group. The elements in Group 16 are:
- Oxygen (O): Crucial for respiration and a vital component of water and countless organic molecules.
- Sulfur (S): Found in various forms, from elemental sulfur to sulfates and sulfides, essential for life and widely used industrially.
- Selenium (Se): A trace element with antioxidant properties, used in some dietary supplements and photocopiers.
- Tellurium (Te): Used in alloys and as a semiconductor.
- Polonium (Po): A rare and highly radioactive element, with limited practical applications.
- Livermorium (Lv): A synthetic, extremely radioactive element with limited understanding of its properties.
Why Six Valence Electrons? The Electron Configuration
The six valence electrons in Group 16 elements arise directly from their electron configuration. The general electron configuration for Group 16 elements is ns²np⁴, where 'n' represents the principal quantum number corresponding to the outermost shell. For example:
- Oxygen (O): 1s²2s²2p⁴ (2 valence electrons in the 2s subshell and 4 in the 2p subshell)
- Sulfur (S): 1s²2s²2p⁶3s²3p⁴ (2 valence electrons in the 3s subshell and 4 in the 3p subshell)
This configuration shows that the outermost shell is not completely filled. To achieve a stable octet, Group 16 elements tend to gain two electrons, forming anions with a -2 charge (e.g., O²⁻, S²⁻). This tendency to gain electrons explains their generally high electronegativity, meaning they have a strong attraction for electrons in a chemical bond.
Chemical Bonding and the Implications of Six Valence Electrons
The presence of six valence electrons significantly influences how Group 16 elements bond with other atoms. They readily form ionic bonds with metals, accepting two electrons to achieve a stable octet. For instance, the formation of magnesium oxide (MgO) involves magnesium (Mg) donating two electrons to an oxygen atom (O), resulting in Mg²⁺ and O²⁻ ions that are electrostatically attracted to each other.
However, Group 16 elements also form covalent bonds with non-metals, sharing electrons to satisfy the octet rule. This is evident in molecules like oxygen (O₂), sulfur dioxide (SO₂), and water (H₂O). In these molecules, the atoms share electrons to achieve a more stable electron configuration, with each atom surrounding itself with eight electrons.
The ability to form both ionic and covalent bonds contributes to the remarkable versatility of Group 16 elements and their presence in a wide range of compounds.
Periodic Trends and the Impact on Group 16 Properties
As you move down Group 16, several periodic trends significantly impact the properties of the elements:
- Atomic Radius: The atomic radius increases down the group due to the addition of electron shells. This larger size leads to decreased electronegativity and ionization energy.
- Electronegativity: Electronegativity decreases down the group. Oxygen is the most electronegative element in Group 16, while the electronegativity of polonium is considerably lower.
- Ionization Energy: Ionization energy decreases down the group, meaning it requires less energy to remove an electron from larger atoms.
- Melting and Boiling Points: These properties generally increase down the group, reflecting the stronger interatomic forces in the heavier chalcogens.
These trends explain the gradual shift in properties observed within Group 16. Oxygen and sulfur are non-metals with characteristically different properties compared to tellurium and polonium, which exhibit some metalloid or even metallic characteristics.
The Diverse Roles of Group 16 Elements
Group 16 elements are essential components of our planet and play crucial roles in various biological and industrial processes:
- Oxygen (O): Essential for respiration in most living organisms, it's a vital component of water and organic molecules. Its high reactivity makes it crucial in combustion processes.
- Sulfur (S): Important for the synthesis of certain amino acids and proteins. It’s used in the vulcanization of rubber, the production of sulfuric acid (a vital industrial chemical), and in the manufacture of fertilizers.
- Selenium (Se): A trace element with antioxidant properties, beneficial for human health. It's used in photocopiers and some photovoltaic cells.
- Tellurium (Te): Used in alloys to improve their machinability and in some semiconductor applications.
- Polonium (Po): Due to its high radioactivity, polonium has limited applications, mostly in specialized research areas.
Conclusion: The Significance of Six Valence Electrons
The simple answer to the question, "How many valence electrons does Group 16 have?" is six. However, understanding the implications of this number unlocks a wealth of knowledge about the chemical behavior, bonding characteristics, and the remarkable diversity of the chalcogens. Their six valence electrons drive their reactivity, influence their bonding patterns, and ultimately contribute to their widespread presence and importance in the natural world and various applications. From the air we breathe to the industrial processes that shape our world, Group 16 elements play a crucial and multifaceted role. Further exploring their properties and applications provides valuable insight into the fundamental principles of chemistry and the power of periodic trends.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Right Triangle
Mar 16, 2025
-
Which Of The Following Is A Function Of A Nucleus
Mar 16, 2025
-
What Is The Positive Square Root Of 25
Mar 16, 2025
-
How Many Sides In A Parallelogram
Mar 16, 2025
-
Whats The Lcm Of 9 And 15
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Group 16 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
