How Many Valence Electrons Does Cu Have
Juapaving
Mar 15, 2025 · 5 min read
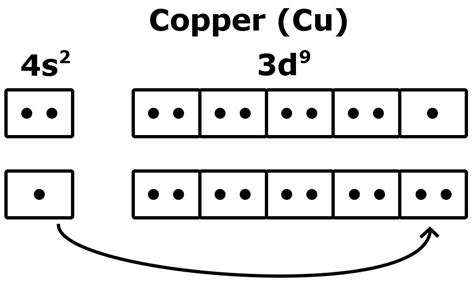
Table of Contents
How Many Valence Electrons Does Cu Have? Delving into Copper's Electronic Structure
Copper (Cu), a reddish-orange metal known for its excellent electrical conductivity and malleability, plays a crucial role in various industries and biological processes. Understanding its electronic structure, particularly the number of valence electrons, is fundamental to comprehending its properties and behavior. This article will delve deep into the electronic configuration of copper, explaining why determining its valence electrons isn't as straightforward as it might seem for other elements, and exploring the implications of its unique electron arrangement.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in copper, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of chemical bonds it can form (ionic, covalent, metallic). The number of valence electrons typically dictates an element's group number in the periodic table.
Copper's Electronic Configuration: The Anomaly
Copper's atomic number is 29, meaning it has 29 protons and 29 electrons in a neutral atom. Following the Aufbau principle (filling orbitals in order of increasing energy), one might expect the electronic configuration of copper to be 1s²2s²2p⁶3s²3p⁶4s²3d⁹. However, this is not the case. The actual electronic configuration of copper is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰.
This seemingly minor difference – a single electron shifting from the 4s orbital to the 3d orbital – has significant consequences. The reason for this anomaly lies in the stability conferred by a completely filled d subshell. A completely filled or half-filled subshell represents a state of higher stability due to electron-electron repulsions being minimized and enhanced exchange energy. Moving one electron from the 4s to the 3d orbital creates a more stable configuration for copper.
Determining Copper's Valence Electrons: The Debate
This unusual electronic configuration leads to a debate about the number of valence electrons in copper. While the simple answer based on the outermost shell (4s) would suggest one valence electron, considering the involvement of the 3d electrons in chemical bonding complicates matters.
Arguments for One Valence Electron:
- Outermost shell: The 4s electron is the electron furthest from the nucleus and, therefore, the most likely to participate in chemical bonding.
- Ionic bonding: Copper often forms +1 ions (Cu⁺) by losing the single 4s electron.
Arguments for Two Valence Electrons:
- 3d orbital participation: The 3d electrons are relatively close in energy to the 4s electron and can participate in chemical bonding, especially in compounds where copper exhibits a +2 oxidation state (Cu²⁺). These compounds are quite common.
- Covalent bonding: In some covalent compounds, both the 4s and 3d electrons may be involved in bond formation.
The Practical Perspective: Considering Oxidation States
The most pragmatic approach to determining the number of valence electrons for copper is to consider its oxidation states. Copper exhibits two common oxidation states: +1 (cuprous) and +2 (cupric).
-
Cu⁺ (Cuprous): In this state, copper loses one electron, implying one valence electron. This is observed in compounds like cuprous oxide (Cu₂O) and cuprous chloride (CuCl).
-
Cu²⁺ (Cupric): In this state, copper loses two electrons, suggesting two valence electrons. This is prevalent in compounds like cupric oxide (CuO) and cupric sulfate (CuSO₄).
Therefore, instead of assigning a fixed number of valence electrons, it’s more accurate to state that copper typically has one or two valence electrons, depending on its oxidation state and the specific chemical environment.
Implications of Copper's Electronic Structure
The unusual electronic configuration and variable valence electrons of copper significantly influence its properties and reactivity.
Electrical Conductivity:
The readily available 4s and 3d electrons contribute to copper's exceptional electrical conductivity. These electrons are relatively loosely held and can move freely throughout the metallic lattice, allowing for easy electron flow and efficient charge transfer.
Catalytic Activity:
The ability of copper to exist in multiple oxidation states makes it an excellent catalyst in various chemical reactions. It can easily accept and donate electrons, facilitating redox reactions (reduction-oxidation reactions). This property is exploited in numerous industrial catalytic processes.
Biological Role:
Copper is an essential trace element for many living organisms. It plays a vital role in enzymatic processes as a redox cofactor, facilitating electron transfer in biological systems. Its variable valence state allows it to participate in a range of important biological reactions.
Alloy Formation:
Copper's ability to form alloys with other metals is a consequence of its electronic structure. The readily available valence electrons allow copper to form strong metallic bonds with other elements, leading to materials with enhanced properties such as strength, hardness, and corrosion resistance. Brass (copper and zinc) and bronze (copper and tin) are classic examples of such alloys.
Conclusion: Understanding the Nuances
While a simplistic answer to "How many valence electrons does Cu have?" might be tempting, the reality is more nuanced. Copper's unique electronic configuration leads to variable valence electron behavior, primarily one or two, depending on its oxidation state and the nature of the chemical bond. Understanding this variability is key to comprehending its diverse properties and wide range of applications in various fields, from electrical engineering to biological systems. The seemingly anomalous electron configuration ultimately contributes to copper's unique and valuable characteristics. This understanding extends beyond simple memorization; it allows for a deeper appreciation of the complexities within the periodic table and the subtle yet profound differences in elemental behavior.
Latest Posts
Latest Posts
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Cu Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
