How Many Valence Electrons Are In P
Juapaving
Mar 20, 2025 · 5 min read
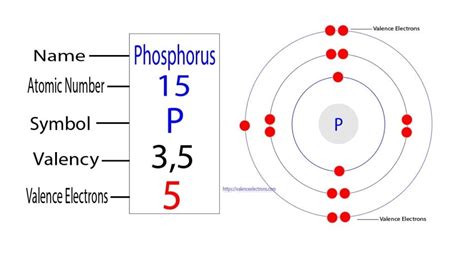
Table of Contents
How Many Valence Electrons Are in Phosphorus (P)? Understanding Valence Electrons and Their Importance
Phosphorus (P), a crucial element for life, plays a vital role in biological processes and industrial applications. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping its chemical behavior and reactivity. This comprehensive guide delves into the intricacies of phosphorus' valence electrons, explaining their determination, significance, and implications for the element's properties and compounds.
What are Valence Electrons?
Before we dive into the specifics of phosphorus, let's establish a fundamental understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the most loosely bound and therefore play the most significant role in chemical bonding and reactions. They determine an atom's reactivity, its ability to form bonds with other atoms, and the types of compounds it can form. The number of valence electrons directly influences an element's oxidation state and its position in the periodic table.
Determining the Number of Valence Electrons in Phosphorus
Phosphorus is located in Group 15 (also known as Group VA) of the periodic table. Group 15 elements are characterized by having five valence electrons. This is a direct consequence of phosphorus' electron configuration.
Electron Configuration of Phosphorus
The atomic number of phosphorus is 15, meaning it has 15 protons and 15 electrons in a neutral atom. The electron configuration of phosphorus is 1s²2s²2p⁶3s²3p³. Let's break this down:
- 1s²: Two electrons in the first energy level (shell).
- 2s²: Two electrons in the second energy level.
- 2p⁶: Six electrons in the second energy level's p subshell.
- 3s²: Two electrons in the third energy level.
- 3p³: Three electrons in the third energy level's p subshell.
The outermost shell for phosphorus is the third energy level (n=3). This shell contains a total of five electrons (two from the 3s subshell and three from the 3p subshell). Therefore, phosphorus has five valence electrons.
The Significance of Phosphorus' Five Valence Electrons
The presence of five valence electrons significantly influences phosphorus' chemical behavior and the types of bonds it can form. This leads to several key characteristics:
1. Covalent Bonding: The Dominant Bonding Type for Phosphorus
Because phosphorus needs three more electrons to achieve a stable octet (eight electrons in its outermost shell), it readily forms covalent bonds. It shares its five valence electrons with other atoms to complete its octet, resulting in the formation of various covalent compounds. Examples include phosphorus trichloride (PCl₃), phosphorus pentachloride (PCl₅), and various phosphorus oxides (P₄O₁₀, P₂O₅).
2. Variable Oxidation States
The five valence electrons allow phosphorus to exhibit variable oxidation states. This means it can lose, gain, or share electrons to achieve different oxidation states depending on the bonding partner and reaction conditions. Common oxidation states for phosphorus include -3, +3, and +5. The versatility in oxidation states contributes to the diverse chemistry of phosphorus.
3. Formation of Different Allotropes
Phosphorus exists in several allotropic forms, each with different physical and chemical properties. These allotropes arise from the different ways phosphorus atoms can bond to each other using their valence electrons. White phosphorus (P₄), red phosphorus, and black phosphorus are examples of these allotropes, each possessing distinct structures and reactivities. The unique bonding arrangements directly stem from the availability of five valence electrons.
4. Importance in Biological Systems
The five valence electrons contribute to phosphorus' essential role in biological systems. Phosphorus is a crucial component of ATP (adenosine triphosphate), the primary energy currency of cells. Its ability to form stable phosphate bonds is essential for energy storage and transfer. Phosphorus is also a component of DNA and RNA, the genetic material of living organisms. The strong covalent bonds involving phosphorus ensure the stability of these vital molecules.
Phosphorus' Compounds and Their Relevance
The chemical behavior of phosphorus, dictated by its five valence electrons, leads to the formation of a wide array of compounds with diverse applications. Let's explore some key examples:
1. Phosphates
Phosphates (PO₄³⁻) are anionic species derived from phosphoric acid (H₃PO₄). They are widely used in fertilizers due to their essential role in plant growth. The strong P-O bonds contribute to the stability of phosphate compounds, making them readily available to plants. Phosphates are also found in detergents, food additives, and various industrial applications.
2. Phosphides
Phosphides are compounds containing phosphorus anions (P³⁻). They are often formed with metals and exhibit a range of properties depending on the metal involved. Some phosphides are semiconductors, while others have applications in materials science and catalysis. The bonding nature in phosphides is crucial for their electronic and structural properties.
3. Organophosphorus Compounds
Organophosphorus compounds contain carbon-phosphorus bonds. These compounds have found wide applications in various fields, including pesticides, nerve agents (some are highly toxic), flame retardants, and pharmaceuticals. The bonding between carbon and phosphorus, enabled by the valence electrons, determines the properties and functions of these diverse compounds. Understanding the electronic structure of organophosphorus compounds is crucial for their design, synthesis, and application.
Beyond the Basics: Delving Deeper into Phosphorus Chemistry
The seemingly simple concept of five valence electrons unlocks a complex and fascinating world of phosphorus chemistry. Further exploration into the field reveals nuances and complexities:
- Bonding Orbitals: A more advanced understanding of phosphorus' bonding involves considering the hybridization of atomic orbitals (sp³, sp³d, sp³d²). These hybrid orbitals explain the different geometries of phosphorus-containing molecules and their reactivity.
- Redox Chemistry: The variable oxidation states of phosphorus lead to rich redox chemistry. Phosphorus can act as both an oxidizing and reducing agent, participating in a wide variety of oxidation-reduction reactions.
- Spectroscopy: Techniques like NMR (Nuclear Magnetic Resonance) and XPS (X-ray Photoelectron Spectroscopy) are crucial for characterizing phosphorus compounds and elucidating their structure and bonding.
Conclusion
Phosphorus, with its five valence electrons, holds a pivotal position in the world of chemistry and biology. These electrons dictate the element's reactivity, bonding characteristics, and the formation of diverse compounds with significant implications across various fields. From fertilizers and detergents to biological molecules and pharmaceuticals, the impact of phosphorus' five valence electrons is undeniable. This comprehensive overview provides a foundation for understanding the fundamental principles underpinning phosphorus' unique and essential role in the world around us. Further exploration into its rich chemistry reveals an even more intricate tapestry of scientific knowledge and technological applications.
Latest Posts
Latest Posts
-
How Many Minutes Are In A Month
Mar 20, 2025
-
What Is The Lcm Of 7 And 8
Mar 20, 2025
-
What Electrical Charge Does Dna Have
Mar 20, 2025
-
Is Water An Element Or Compound
Mar 20, 2025
-
Lcm Of 4 6 And 8
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In P . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
