How Many Unpaired Electrons Does Fe Have
Juapaving
May 12, 2025 · 5 min read
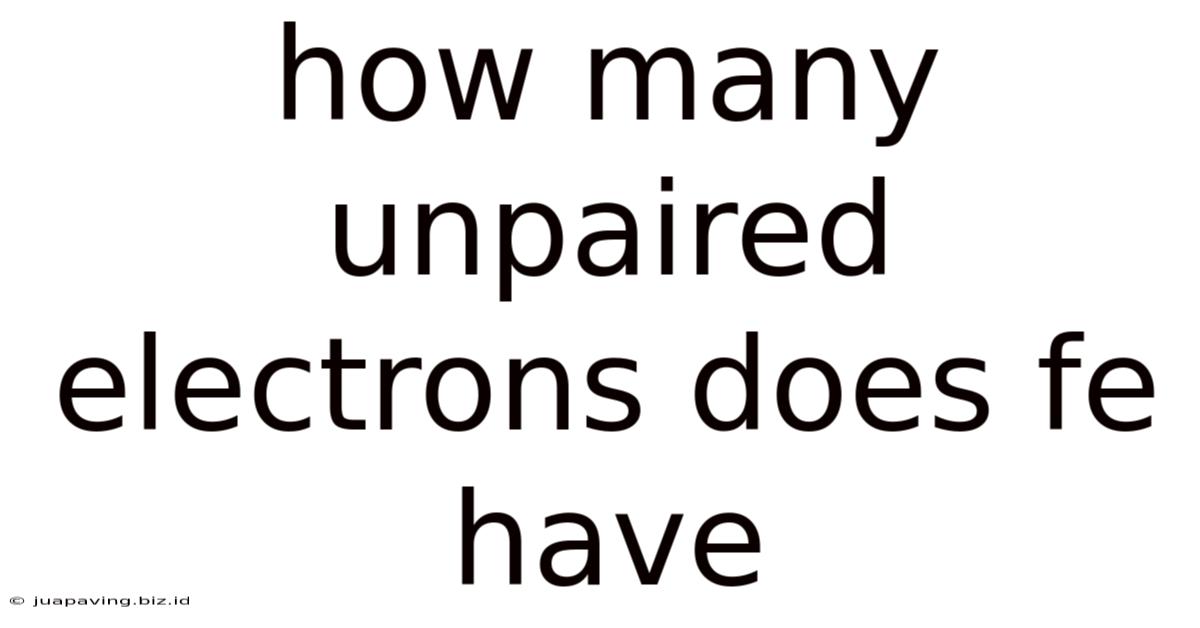
Table of Contents
How Many Unpaired Electrons Does Fe Have? A Deep Dive into Iron's Electronic Configuration
Iron (Fe), a ubiquitous element crucial for life and industry, possesses a fascinating electronic structure that dictates its remarkable properties. Understanding its electron configuration is key to grasping its magnetic behavior, reactivity, and diverse applications. This article delves into the intricacies of iron's electronic structure, specifically addressing the question: How many unpaired electrons does Fe have? We'll explore the underlying principles of electron configuration, delve into the specifics of iron's various oxidation states, and consider the implications of its unpaired electrons.
Understanding Electron Configuration and Hund's Rule
Before we pinpoint the number of unpaired electrons in iron, let's establish the foundational concepts. Electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. This distribution follows specific rules, most importantly the Aufbau principle (electrons fill orbitals from lowest to highest energy) and Hund's rule.
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons, being negatively charged, repel each other. They prefer to occupy separate orbitals within a subshell, minimizing electron-electron repulsion and achieving a lower overall energy state. This tendency towards maximizing spin multiplicity (having as many unpaired electrons as possible) is crucial for understanding the magnetic properties of atoms like iron.
Iron's Electronic Configuration: The Ground State
Iron has an atomic number of 26, meaning it has 26 protons and, in its neutral state, 26 electrons. Its ground state electron configuration is written as: 1s²2s²2p⁶3s²3p⁶4s²3d⁶.
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the completely filled inner shells, with all electrons paired. These electrons are relatively tightly bound to the nucleus and don't significantly contribute to iron's chemical reactivity or magnetic properties.
-
4s²: The 4s subshell is filled with two paired electrons.
-
3d⁶: This is where things get interesting. The 3d subshell has five orbitals, each capable of holding two electrons. According to Hund's rule, the six 3d electrons in iron will initially occupy each of the five 3d orbitals individually, with one orbital containing a pair. This leaves four unpaired electrons in the 3d subshell.
Therefore, in its ground state, iron (Fe) has four unpaired electrons.
Iron's Oxidation States and Unpaired Electrons
Iron exhibits variable oxidation states, most commonly +2 (ferrous) and +3 (ferric). The number of unpaired electrons can change depending on the oxidation state:
Fe²⁺ (Ferrous Ion):
When iron loses two electrons to become Fe²⁺, it typically loses the two electrons from the 4s subshell. This leaves the 3d subshell with six electrons. Following Hund's rule, this results in four unpaired electrons in the Fe²⁺ ion.
Fe³⁺ (Ferric Ion):
In the Fe³⁺ ion, iron loses three electrons. While it's commonly assumed that the 4s electrons and one 3d electron are lost, the actual process is more complex and involves electron-electron interactions. Regardless of the precise electron removal order, the result is a 3d⁵ electron configuration. Following Hund's rule, this gives five unpaired electrons in the Fe³⁺ ion.
The Significance of Unpaired Electrons in Iron's Properties
The presence of unpaired electrons is responsible for many of iron's crucial properties:
-
Ferromagnetism: Iron is a ferromagnetic material, meaning it exhibits a strong attraction to magnetic fields and can be permanently magnetized. This ferromagnetism arises from the interaction between the unpaired electrons in multiple iron atoms. The unpaired electrons' spins align parallel in domains, creating a net magnetic moment.
-
Catalysis: Iron's unpaired electrons play a vital role in its catalytic activity. These electrons can participate in redox reactions (reduction-oxidation reactions), facilitating the breaking and formation of chemical bonds. This is crucial in various industrial processes and biological systems. For instance, iron is a crucial component of many enzymes involved in crucial biological processes.
-
Color: The transition metal nature of iron, with its partially filled d orbitals, contributes to its characteristic colors in different compounds. The unpaired electrons and their interaction with light are responsible for the absorption and emission of specific wavelengths, resulting in the observed color. For example, ferrous compounds often display a greenish hue while ferric compounds tend toward brownish or yellowish colors.
Beyond the Basics: More Complex Considerations
While the basic electron configuration provides a good starting point, the actual electronic structure of iron in various compounds and environments can be more complex. Factors such as ligand field effects (the influence of surrounding atoms or molecules on the electron configuration) can significantly alter the number and arrangement of unpaired electrons.
For instance, in coordination complexes, the ligands surrounding the iron ion can influence the splitting of the d orbitals, potentially leading to a change in the number of unpaired electrons. This is a key concept in crystal field theory and ligand field theory.
Conclusion: Iron's Unpaired Electrons - A Foundation for its Functionality
The number of unpaired electrons in iron is not a static value; it depends on its oxidation state and the specific chemical environment. However, the general principle remains: iron, in its common oxidation states, possesses a significant number of unpaired electrons. These unpaired electrons are fundamentally responsible for its ferromagnetism, catalytic activity, and characteristic colors. Understanding the electronic configuration and the implications of unpaired electrons is essential for appreciating the diverse and crucial roles iron plays in both nature and technology. Further exploration into more complex aspects like ligand field theory allows for a deeper understanding of iron's behavior in diverse chemical environments. This intricate interplay of electrons defines the fundamental properties of this incredibly important element.
Latest Posts
Latest Posts
-
What Percentage Is 3 Of 16
May 13, 2025
-
What Are The Properties Of Numbers
May 13, 2025
-
What Are Three Properties Of Acids
May 13, 2025
-
What Fraction Is Equal To 4 5
May 13, 2025
-
Which Of The Following Is An Example Of A Disaccharide
May 13, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Fe Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.