How Many Protons Do Sodium Have
Juapaving
Mar 30, 2025 · 5 min read
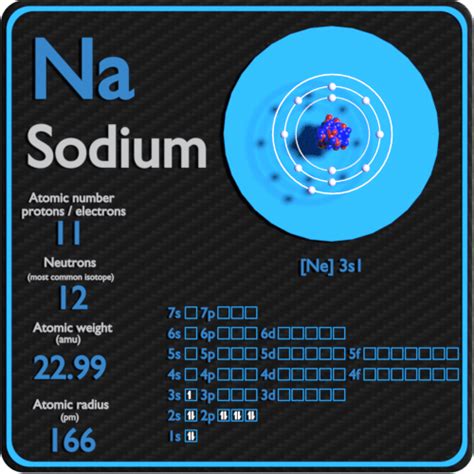
Table of Contents
How Many Protons Does Sodium Have? Understanding Atomic Structure and Sodium's Properties
Sodium, a ubiquitous element crucial to life and numerous industrial processes, holds a fascinating place in the periodic table. Understanding its fundamental properties, especially its atomic structure, is key to grasping its behavior and applications. A central aspect of this understanding revolves around a simple yet fundamental question: How many protons does sodium have?
Delving into Atomic Structure: Protons, Neutrons, and Electrons
Before we answer the titular question, let's lay the groundwork by briefly reviewing atomic structure. An atom, the fundamental building block of matter, is composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's identity – it's the atomic number.
- Neutrons: Neutrally charged particles also found within the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The arrangement of these particles, particularly the number of protons and electrons, dictates an element's chemical properties and reactivity. The nucleus, containing the protons and neutrons, holds almost all of the atom's mass. The electrons, comparatively much lighter, determine the atom's ability to form chemical bonds and interact with other atoms.
Sodium's Atomic Number: The Key to its Identity
The atomic number of an element is equivalent to the number of protons in its nucleus. This number uniquely identifies the element and its place on the periodic table. For sodium (Na), the atomic number is 11.
Therefore, the definitive answer to the question "How many protons does sodium have?" is 11. This single fact unlocks a wealth of information about sodium's properties and behavior.
Isotopes of Sodium: Variations in Neutron Count
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with differing neutron counts are called isotopes. Sodium has several isotopes, the most common being Sodium-23 (²³Na), which comprises over 99.9% of naturally occurring sodium. This means that most sodium atoms have 11 protons and 12 neutrons (23 - 11 = 12).
Other isotopes of sodium, such as Sodium-22 (²²Na) and Sodium-24 (²⁴Na), are less abundant and often radioactive. These isotopes have different numbers of neutrons, altering their stability and decay properties. While the neutron count influences an isotope's properties like mass and radioactivity, the number of protons remains constant at 11, solidifying its identity as sodium.
Sodium's Properties: A Consequence of its 11 Protons
Sodium's properties are directly linked to its atomic structure, especially its 11 protons and the resulting electron configuration. The single electron in its outermost shell makes it highly reactive, readily losing this electron to achieve a stable octet configuration. This explains its:
- High reactivity: Sodium readily reacts with water, oxygen, and other elements, often violently.
- Low ionization energy: It requires relatively little energy to remove the outer electron.
- Metallic character: Sodium is a soft, silvery-white metal, exhibiting characteristic metallic properties such as conductivity.
- Formation of ionic compounds: Sodium readily forms ionic bonds, losing an electron to become a positively charged ion (Na⁺). This ionic nature contributes to the formation of many essential compounds, such as sodium chloride (table salt).
Sodium's Significance in Biology and Industry
Sodium's unique properties make it incredibly significant in various aspects of life and technology:
Biological Importance:
- Electrolyte balance: Sodium ions (Na⁺) play a vital role in maintaining the electrolyte balance in the human body, crucial for nerve impulse transmission, muscle contraction, and fluid regulation.
- Nutrient: It's an essential nutrient, albeit in moderate amounts, contributing to various bodily functions.
Industrial Applications:
- Sodium chloride (NaCl): Common table salt, is a cornerstone of numerous industrial processes, ranging from food preservation to chemical manufacturing.
- Sodium hydroxide (NaOH): A strong alkali, used extensively in various industries, including soap and paper production.
- Sodium lamps: These lamps emit intense yellow light, making them useful in various applications, from street lighting to high-pressure sodium lamps used in sports stadiums.
- Sodium compounds in pharmaceuticals: Sodium compounds feature prominently in various pharmaceuticals and medicinal applications.
Understanding Sodium's Role Through its Atomic Structure
By understanding that sodium has 11 protons, we gain insight into its characteristic properties and its pivotal role in biological systems and various industries. This fundamental knowledge allows us to appreciate the crucial link between atomic structure and macroscopic behavior. The seemingly simple number of 11 protons dictates the reactivity, bonding patterns, and overall behavior of this essential element.
Exploring Further: The Periodic Table and Atomic Structure
The periodic table organizes elements based on their atomic number, reflecting their properties and relationships. Sodium's position in the table, in Group 1 (alkali metals), highlights its chemical behavior and reactivity, readily predictable based on its 11 protons. Further exploration into the principles of atomic structure, electronic configuration, and chemical bonding provides a richer understanding of sodium and other elements. Understanding isotopic variations also provides insights into the complexities of elemental behavior.
Sodium: A Foundation of Chemistry and Life
In conclusion, the answer to "How many protons does sodium have?" is unequivocally 11. This seemingly small piece of information is fundamental to comprehending sodium's unique characteristics and its significant role in both the natural world and human endeavors. From its biological importance in maintaining electrolyte balance to its widespread industrial applications, the impact of this element, shaped by its atomic structure, is undeniable. The exploration of sodium's 11 protons serves as a gateway to understanding the broader principles of atomic structure and the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Which Is Larger Pound Or Kilogram
Apr 01, 2025
-
Are Histograms And Bar Graphs The Same
Apr 01, 2025
-
How Many Vowels Are There In English Language
Apr 01, 2025
-
Two Angles That Add Up To 90 Degrees
Apr 01, 2025
-
What Is An Analogy For Mitochondria
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Do Sodium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
