How Many Neutrons Does Phosphorus Have
Juapaving
Mar 24, 2025 · 5 min read
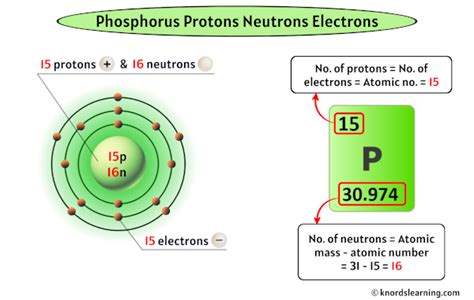
Table of Contents
How Many Neutrons Does Phosphorus Have? Understanding Isotopes and Atomic Structure
Phosphorus, a crucial element for life, presents a fascinating study in atomic structure. A simple question, "How many neutrons does phosphorus have?", reveals a deeper understanding of isotopes and the variations within a single element. This article dives deep into the answer, exploring the concept of atomic number, mass number, isotopes, and the significance of neutron count in phosphorus's properties and applications.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before tackling the neutron count in phosphorus, let's review the fundamental building blocks of an atom:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutrally charged particles also found in the nucleus. Their number contributes to the atom's mass but doesn't alter its chemical identity.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. They determine the atom's chemical reactivity and bonding behavior.
Phosphorus: Atomic Number and the Standard Isotope
Phosphorus (P) has an atomic number of 15. This means every phosphorus atom possesses 15 protons. This number is constant and unchangeable for all phosphorus atoms. Changing the number of protons fundamentally changes the element itself.
The most common isotope of phosphorus, phosphorus-31 (³¹P), accounts for nearly 100% of naturally occurring phosphorus. This notation, ³¹P, indicates the mass number of the isotope. The mass number is the sum of protons and neutrons.
Therefore, to find the number of neutrons in phosphorus-31, we subtract the atomic number (protons) from the mass number:
31 (mass number) - 15 (protons) = 16 neutrons
So, phosphorus-31 has 16 neutrons.
Isotopes of Phosphorus: Variations in Neutron Count
While phosphorus-31 is overwhelmingly dominant, other isotopes of phosphorus exist, albeit with significantly lower abundances. These isotopes differ in their neutron count but share the same number of protons (15). Some examples include:
-
Phosphorus-32 (³²P): This isotope has 17 neutrons (32 - 15 = 17). It's radioactive and used as a tracer in biological and medical research. Its relatively short half-life makes it useful for tracking processes within a short timeframe.
-
Phosphorus-33 (³³P): With 18 neutrons (33 - 15 = 18), this isotope is also radioactive but has a longer half-life than ³²P. Its applications often involve longer-term studies.
-
Phosphorus-34 (³⁴P): Possessing 19 neutrons (34-15=19), this is another radioactive isotope with a considerably shorter half-life than ³³P.
The existence of these isotopes demonstrates that the neutron count in phosphorus can vary. While phosphorus-31 is the standard and most abundant form, understanding the other isotopes is vital, particularly in fields like nuclear medicine and radioactive dating.
The Significance of Neutron Count in Phosphorus's Properties
The neutron count significantly impacts an atom's properties, although not as dramatically as the proton count. The primary effect is on the atom's mass and stability.
Mass and Density:
A higher neutron count leads to a higher atomic mass, influencing the overall density of the material containing phosphorus. This variation, though subtle in phosphorus's case, is more pronounced in heavier elements.
Nuclear Stability and Radioactivity:
The neutron-to-proton ratio is crucial for nuclear stability. Isotopes with an imbalanced ratio are often radioactive, meaning they decay over time, emitting radiation. Phosphorus-32 and phosphorus-33 are prime examples of radioactive isotopes due to their imbalanced neutron-to-proton ratios. This radioactivity makes them useful in specific applications like medical imaging and tracing biochemical pathways, but also necessitates careful handling.
Chemical Properties:
While the number of neutrons doesn't change the element's chemical properties (those determined by the electrons), subtle differences in isotopic mass can affect reaction rates in certain processes. These isotope effects are typically small but measurable, especially in reactions involving bond breaking or formation.
Applications of Phosphorus and its Isotopes
Phosphorus, in its various forms, plays a critical role in numerous fields:
Biological Significance:
Phosphorus is an essential element for life. It is a crucial component of DNA and RNA, the genetic building blocks of all living organisms. It’s also a key element in ATP (adenosine triphosphate), the primary energy currency of cells. The stable phosphorus-31 isotope is vital in all these biological functions.
Agricultural Use:
Phosphorus is a primary nutrient in fertilizers. Its availability in soil directly impacts plant growth and crop yield. Understanding the different forms and availability of phosphorus in the soil is crucial for sustainable agriculture.
Industrial Applications:
Phosphorus compounds find use in various industrial processes, including the production of detergents, pesticides, and specialized glasses.
Medical and Research Applications:
Radioactive isotopes of phosphorus, like phosphorus-32, play a critical role in medical research and treatment. They're used as tracers to study metabolic pathways, diagnose and treat certain cancers, and in various radiolabeling techniques.
Conclusion: Beyond a Simple Number
The question of how many neutrons phosphorus has isn't merely a simple numerical answer. Understanding the variations in neutron count across phosphorus isotopes provides insights into nuclear stability, atomic structure, and the diverse applications of this essential element. While phosphorus-31, with its 16 neutrons, represents the most common and stable form, the radioactive isotopes, with their differing neutron numbers, have found critical applications in medicine and scientific research. This exploration highlights the intricate relationship between atomic structure, isotopic variations, and the element's multifaceted role in the natural world and human applications.
Latest Posts
Latest Posts
-
Least Common Multiple Of 48 And 72
Mar 30, 2025
-
2 Similarities Between Dna And Rna
Mar 30, 2025
-
Where Is The Circumcenter Of A Right Triangle Located
Mar 30, 2025
-
A Horizontal Row On The Periodic Table Is Called
Mar 30, 2025
-
Quotient Of 80 Divided By 5
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Phosphorus Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
