How Many Neutrons Are In Mg
Juapaving
Apr 06, 2025 · 5 min read
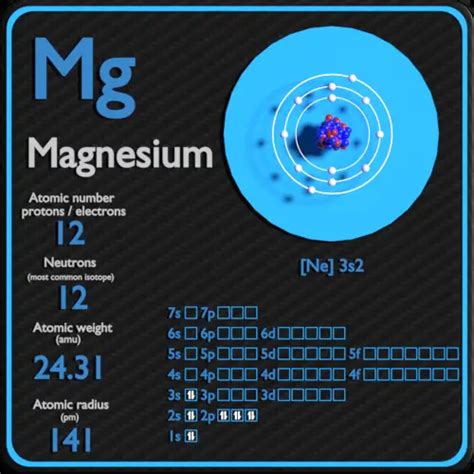
Table of Contents
How Many Neutrons Are in Mg? Understanding Magnesium Isotopes and Neutron Count
Magnesium (Mg), a vital element for plant and animal life, presents a fascinating study in isotopic variation. Understanding the number of neutrons in magnesium requires delving into the concept of isotopes and their prevalence in nature. This article will comprehensively explore the different magnesium isotopes, their neutron counts, and the implications of this variation in various fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the specifics of magnesium isotopes, let's establish a fundamental understanding of atomic structure. Every atom consists of three primary subatomic particles:
-
Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and its identity on the periodic table. Magnesium's atomic number is 12, meaning every magnesium atom has 12 protons.
-
Neutrons: Neutrally charged particles also residing in the atom's nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the existence of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus. In a neutral atom, the number of electrons equals the number of protons.
Isotopes: Variations in Neutron Count
Isotopes are atoms of the same element that possess the same number of protons but differ in their neutron count. This difference in neutron number affects the atom's mass but not its chemical properties. Magnesium has three naturally occurring stable isotopes:
-
Magnesium-24 (²⁴Mg): This is the most abundant isotope of magnesium, making up approximately 79% of naturally occurring magnesium. It has 12 protons and 12 neutrons. (24 - 12 = 12)
-
Magnesium-25 (²⁵Mg): This isotope accounts for about 10% of naturally occurring magnesium. It contains 12 protons and 13 neutrons. (25 - 12 = 13)
-
Magnesium-26 (²⁶Mg): The least abundant stable isotope of magnesium, comprising roughly 11% of naturally occurring magnesium. It possesses 12 protons and 14 neutrons. (26 - 12 = 14)
The number after the element's name (e.g., 24, 25, 26) represents the mass number, which is the sum of protons and neutrons in the atom's nucleus. Therefore, by subtracting the atomic number (number of protons) from the mass number, we can determine the number of neutrons in each isotope.
Calculating Neutron Count: A Step-by-Step Guide
To calculate the number of neutrons in any isotope, follow these simple steps:
-
Identify the mass number: This number is usually written as a superscript to the left of the element's symbol (e.g., ²⁴Mg).
-
Find the atomic number: The atomic number is the number of protons and is found on the periodic table. For magnesium, it is 12.
-
Subtract the atomic number from the mass number: This difference represents the number of neutrons.
Example: Let's calculate the number of neutrons in Magnesium-26 (²⁶Mg):
- Mass number: 26
- Atomic number (Magnesium): 12
- Number of neutrons: 26 - 12 = 14
Therefore, Magnesium-26 has 14 neutrons.
The Significance of Isotopic Variations in Magnesium
The presence of multiple stable isotopes of magnesium has significant implications across various scientific disciplines:
-
Geology and Geochemistry: The isotopic ratios of magnesium in rocks and minerals provide valuable insights into geological processes, including magma formation, metamorphic events, and the age of geological formations. Analyzing the relative abundances of ²⁴Mg, ²⁵Mg, and ²⁶Mg can reveal crucial information about the Earth's history.
-
Cosmochemistry: Isotopic ratios of magnesium are also crucial in studying extraterrestrial materials like meteorites. Analyzing these ratios can help scientists understand the origin and evolution of the solar system and the processes that formed planets.
-
Biology and Medicine: Magnesium plays a critical role in various biological processes, and its isotopic composition can be used in biological studies. Stable isotopes are increasingly used as tracers in metabolic studies and for understanding the uptake and distribution of magnesium in living organisms.
-
Nuclear Physics and Applications: While the stable isotopes of magnesium are not radioactive, the study of their nuclear properties contributes to our understanding of nuclear structure and interactions. Furthermore, certain radioactive isotopes of magnesium are used in specific nuclear applications, although not as commonly as isotopes of other elements.
Beyond Stable Isotopes: Radioactive Magnesium Isotopes
While the three isotopes discussed above are stable, several radioactive magnesium isotopes exist. These isotopes are unstable and undergo radioactive decay, transforming into other elements over time. These radioactive isotopes have specific applications in research, but their use is often limited due to their short half-lives and potential hazards.
Conclusion: A Comprehensive Understanding of Magnesium Isotopes and Neutrons
This comprehensive article explored the concept of isotopes and applied it to magnesium, a vital element in various systems. We have learned that magnesium's three naturally occurring isotopes – ²⁴Mg, ²⁵Mg, and ²⁶Mg – contain 12, 13, and 14 neutrons, respectively. Understanding the number of neutrons in these isotopes is crucial for various scientific and practical applications, from geological dating to biological research and nuclear applications. The relative abundance of each isotope and their variations in natural samples provide invaluable insights into various processes that shape the Earth and the cosmos. This detailed explanation provides a foundation for further exploration of this fascinating aspect of chemistry and the natural world. By understanding the intricacies of atomic structure and isotopic variations, we gain a deeper appreciation for the complexity and diversity of matter around us. The knowledge gained from studying isotopes like those of magnesium continues to contribute to advancements in diverse fields, pushing the boundaries of scientific understanding and technological innovation. Further research into magnesium isotopes and their applications remains a vibrant and exciting area of scientific inquiry.
Latest Posts
Latest Posts
-
3 Meters Is How Many Centimeters
Apr 07, 2025
-
Equilateral Triangle Inscribed In A Circle
Apr 07, 2025
-
What Is Larger 2 8 Or 3 4
Apr 07, 2025
-
16 Meters Is How Many Feet
Apr 07, 2025
-
Words With A Z In It
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Mg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
