How Many Lone Pairs In Co2
Juapaving
Mar 15, 2025 · 5 min read
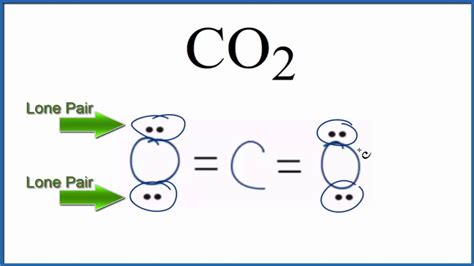
Table of Contents
How Many Lone Pairs in CO₂? Unraveling the Lewis Structure of Carbon Dioxide
Carbon dioxide (CO₂) is a ubiquitous molecule found throughout our environment, playing a crucial role in various natural processes and human activities. Understanding its molecular structure, particularly the number of lone pairs, is fundamental to grasping its chemical behavior and properties. This comprehensive guide delves deep into the Lewis structure of CO₂, explaining how to determine the number of lone pairs and dispelling common misconceptions.
Understanding Lewis Structures: The Foundation of Molecular Geometry
Before we tackle the specific case of CO₂, let's establish a firm understanding of Lewis structures. A Lewis structure, also known as a Lewis dot diagram, is a visual representation of the valence electrons in a molecule. These diagrams help us predict the molecule's geometry, bonding, and overall properties. The key components of a Lewis structure are:
- Valence Electrons: These are the outermost electrons of an atom, which participate in chemical bonding.
- Bonds: Represented by lines connecting atoms, each line represents a shared pair of electrons (a single bond is one shared pair, a double bond is two shared pairs, and a triple bond is three shared pairs).
- Lone Pairs: These are pairs of valence electrons that are not involved in bonding and belong solely to one atom. They are represented by dots.
Knowing how to correctly determine the number of valence electrons and their arrangement is crucial to drawing an accurate Lewis structure.
Determining Valence Electrons for CO₂
To draw the Lewis structure for CO₂, we first need to determine the total number of valence electrons contributed by each atom:
- Carbon (C): Carbon is in group 14 of the periodic table, meaning it has 4 valence electrons.
- Oxygen (O): Oxygen is in group 16, meaning it has 6 valence electrons. Since there are two oxygen atoms, they contribute a total of 6 x 2 = 12 valence electrons.
Therefore, the total number of valence electrons in CO₂ is 4 + 12 = 16.
Constructing the Lewis Structure of CO₂: A Step-by-Step Approach
-
Central Atom Selection: Carbon, being the least electronegative atom, is placed in the center.
-
Connecting Atoms: Connect the carbon atom to each oxygen atom with a single bond. This uses 4 of the 16 valence electrons (2 electrons per bond).
-
Satisfying the Octet Rule: Each oxygen atom needs 8 electrons to satisfy the octet rule (except for Hydrogen which requires only 2 electrons). After the single bonds, each oxygen has 2 electrons from the bond and needs 6 more. This leaves us with 12 remaining electrons.
-
Adding Remaining Electrons: Distribute the remaining 12 electrons as lone pairs around the oxygen atoms. Each oxygen atom receives 3 lone pairs (6 electrons).
-
Octet Check: Check that each atom has a full octet. The oxygen atoms have 8 electrons (2 from the single bond + 6 from the lone pairs), satisfying the octet rule. However, the carbon atom only has 4 electrons.
-
Multiple Bonds: To satisfy the octet rule for carbon, we need to form double bonds with both oxygen atoms. We move two lone pairs from each oxygen atom to form two double bonds with the carbon atom.
The final Lewis structure shows carbon double-bonded to each oxygen atom.
The Final Answer: Zero Lone Pairs on Carbon, Six Lone Pairs Total
After completing the Lewis structure, we can definitively answer the question: How many lone pairs are there in CO₂?
The carbon atom in CO₂ has zero lone pairs. Both oxygen atoms have three lone pairs each, resulting in a total of six lone pairs in the molecule.
Why Understanding Lone Pairs is Crucial
The presence or absence of lone pairs significantly impacts the molecule's shape and properties. Lone pairs exert a stronger repulsive force than bonding pairs, influencing the bond angles and overall molecular geometry.
In the case of CO₂, the absence of lone pairs on the central carbon atom leads to a linear molecular geometry. The double bonds between carbon and oxygen create a symmetrical molecule with a zero dipole moment (meaning it's nonpolar). This linearity and nonpolar nature heavily influence CO₂'s physical and chemical properties, including its solubility, reactivity, and role in climate change.
Common Misconceptions and Clarifications
It's crucial to address some common misconceptions regarding the Lewis structure of CO₂ and the counting of lone pairs:
-
Incorrect Placement of Lone Pairs: Some beginners might incorrectly place lone pairs on the carbon atom, leading to an inaccurate Lewis structure and incorrect lone pair count. Remember, the carbon atom forms double bonds with both oxygen atoms to satisfy the octet rule.
-
Misinterpretation of Double Bonds: Double bonds are crucial in CO₂'s structure. Failure to form double bonds results in an incomplete octet on the carbon atom, violating fundamental principles of chemical bonding.
-
Ignoring the Octet Rule: The octet rule (except for Hydrogen) acts as a guiding principle when drawing Lewis structures. While there are exceptions, it's critical to initially attempt to satisfy the octet rule for each atom.
Beyond the Basics: Delving Deeper into CO₂'s Properties
Understanding the lone pair distribution is just the beginning. This knowledge serves as the foundation for understanding more complex aspects of CO₂:
-
Molecular Geometry and Polarity: The linear shape and absence of a dipole moment contribute to CO₂'s nonpolar nature. This has implications for its interactions with other molecules and its solubility in various solvents.
-
Bonding and Orbital Hybridization: The double bonds in CO₂ result from sp hybridization of the carbon atom. This hybridization significantly influences the bond strength and geometry.
-
Reactivity and Chemical Reactions: The double bonds' electron distribution influences CO₂'s reactivity in various chemical reactions, such as photosynthesis and combustion.
Conclusion: A Comprehensive Understanding of CO₂'s Lone Pairs
In conclusion, the number of lone pairs in CO₂ is critically important for understanding its structure and properties. There are zero lone pairs on the carbon atom and six lone pairs in total (three on each oxygen atom). This understanding is fundamental to interpreting CO₂'s linear geometry, nonpolar nature, and reactivity. This knowledge underpins a broad range of applications, from understanding its role in climate change to its application in industrial processes. Mastering the concept of Lewis structures, valence electrons, and lone pair distribution provides a solid foundation for further exploration of chemical bonding and molecular properties.
Latest Posts
Latest Posts
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
-
Least Common Multiple Of 20 And 3
Mar 17, 2025
-
Function Of The Motor End Plate
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs In Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
