How Many Electrons Can The 4th Energy Level Hold
Juapaving
Mar 16, 2025 · 6 min read
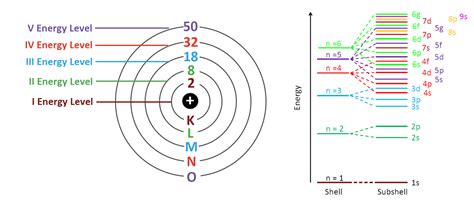
Table of Contents
How Many Electrons Can the 4th Energy Level Hold? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to comprehending the behavior of atoms and the periodic table. A crucial aspect of this understanding involves determining the maximum number of electrons each energy level can accommodate. This article delves into the specifics of the fourth energy level, exploring the principles governing electron capacity and the implications for chemical properties.
Understanding Electron Shells and Subshells
Before diving into the fourth energy level, let's establish the groundwork. Electrons, negatively charged particles, orbit the atom's nucleus in distinct energy levels, often visualized as shells. These shells are not physically defined spaces but represent regions where electrons are most likely to be found. Each shell has a principal quantum number (n), which determines its energy level; n = 1 represents the first shell (closest to the nucleus), n = 2 represents the second, and so on.
However, the story doesn't end with shells. Each shell is further divided into subshells, designated by the letters s, p, d, and f. These subshells represent different shapes and energy sublevels within a given shell. The number of subshells within a shell is equal to its principal quantum number (n).
- s subshell: This subshell has a spherical shape and can hold a maximum of 2 electrons.
- p subshell: This subshell has a dumbbell shape and can hold a maximum of 6 electrons (2 electrons per orbital, with 3 orbitals in total).
- d subshell: This subshell has more complex shapes and can hold a maximum of 10 electrons (2 electrons per orbital, with 5 orbitals in total).
- f subshell: This subshell has even more complex shapes and can hold a maximum of 14 electrons (2 electrons per orbital, with 7 orbitals in total).
The Fourth Energy Level (n=4): Unveiling its Capacity
Now, let's focus on the fourth energy level (n=4). Since n=4, this energy level contains four subshells: 4s, 4p, 4d, and 4f. To determine the total electron capacity of this level, we simply add the maximum electron capacity of each subshell:
- 4s subshell: Holds a maximum of 2 electrons.
- 4p subshell: Holds a maximum of 6 electrons.
- 4d subshell: Holds a maximum of 10 electrons.
- 4f subshell: Holds a maximum of 14 electrons.
Therefore, the total number of electrons the fourth energy level can hold is 2 + 6 + 10 + 14 = 32 electrons.
Why 32 Electrons? A Closer Look at Quantum Mechanics
The capacity of each subshell is dictated by quantum mechanics. The quantum numbers that describe an electron's state – principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms) – govern the allowed energy levels and electron arrangements. The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This principle, along with the rules governing the other quantum numbers, limits the number of electrons that can occupy each subshell and, consequently, the entire energy level.
The 4s subshell, for instance, has n=4 and l=0, leading to only one possible ml value (0) and two possible ms values (+1/2 and -1/2). This results in a maximum of 2 electrons. The 4p subshell has n=4 and l=1, giving rise to three possible ml values (-1, 0, +1) and two possible ms values for each, totaling 6 electrons. Similar reasoning applies to the 4d and 4f subshells, yielding their respective electron capacities.
Implications for Chemical Properties and the Periodic Table
The electron configuration of an element, including the occupancy of its fourth energy level, significantly influences its chemical properties. Elements with partially filled 4s, 4p, 4d, or 4f subshells are particularly reactive, engaging in chemical bonding to achieve a more stable electron configuration (often a filled or half-filled subshell). The transition metals, for example, possess partially filled d orbitals in their outermost energy level, often the 3d or 4d level. This leads to their characteristic variable oxidation states and complex coordination chemistry.
The periodic table is organized based on the electron configurations of elements. The fourth period (row) of the periodic table comprises elements that are progressively filling the 4s, 4p, and 4d subshells. The lanthanides and actinides, occupying the f-block, possess electrons filling the 4f and 5f subshells, respectively. These arrangements are responsible for the chemical similarities and trends observed within the groups and periods of the periodic table.
Beyond the Fourth Energy Level
While this article focuses on the fourth energy level, it's important to remember that higher energy levels exist, with even greater electron capacities. The fifth energy level (n=5), for example, can hold a total of 50 electrons (2 in 5s, 6 in 5p, 10 in 5d, 14 in 5f, and 18 in 5g). The number of electrons in each higher energy level follows the same principles discussed above, determined by the number of subshells and the maximum occupancy of each subshell.
The existence of these higher energy levels and subshells is crucial for understanding the behavior of heavier elements and their complex chemical properties. The filling of these levels involves intricate interactions between electrons and the nucleus, leading to a wide range of chemical and physical properties.
Electron Configuration Examples: Illustrating the Fourth Level's Role
Let's illustrate the role of the fourth energy level in electron configurations with a few examples:
-
Potassium (K): [Ar] 4s<sup>1</sup>. Potassium has one electron in its 4s subshell, making it highly reactive. This single electron readily participates in chemical bonding.
-
Krypton (Kr): [Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>6</sup>. Krypton is a noble gas with a completely filled 4s and 4p subshells. This complete shell structure contributes to its exceptional chemical inertness.
-
Zinc (Zn): [Ar] 3d<sup>10</sup> 4s<sup>2</sup>. Zinc has a full 3d subshell and a full 4s subshell, contributing to its relatively low reactivity compared to other transition metals. Note that the 3d subshell fills before the 4s subshell in this case due to subtle energy level differences.
-
Gadolinium (Gd): [Xe] 4f<sup>7</sup> 5d<sup>1</sup> 6s<sup>2</sup>. Gadolinium exhibits an interesting configuration. It demonstrates the filling of the 4f subshell, which is characteristic of the lanthanides and is a key factor in its magnetic properties.
These examples highlight how the electron configuration, and specifically the occupation of the fourth energy level, directly affects the element's chemical behavior and position within the periodic table.
Conclusion: Mastering Electron Configuration for a Deeper Understanding
Understanding the maximum number of electrons that the fourth energy level can hold – 32 electrons – is fundamental to grasping atomic structure, chemical bonding, and the periodic table’s organization. This knowledge allows for a more profound comprehension of an element's properties and its behavior in chemical reactions. The principles governing electron capacity are rooted in quantum mechanics, emphasizing the importance of understanding quantum numbers and the Pauli Exclusion Principle. By applying this knowledge, we gain invaluable insights into the diverse and fascinating world of chemistry. This foundation enables further exploration into advanced concepts, including molecular orbital theory, spectroscopy, and materials science. Mastering electron configuration unlocks a deeper understanding of the fundamental building blocks of matter and their interactions.
Latest Posts
Latest Posts
-
The Number Of Elements In A Set Is Called The
Mar 17, 2025
-
What Is The Prime Factorization Of 59
Mar 17, 2025
-
What Is The Difference Between A Parallelogram And A Trapezoid
Mar 17, 2025
-
What Is Difference Between Ac Motor And Dc Motor
Mar 17, 2025
-
How Many Cubic Feet Are In A Quart
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The 4th Energy Level Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
