How Many Atoms Are In Cl
Juapaving
Mar 20, 2025 · 5 min read
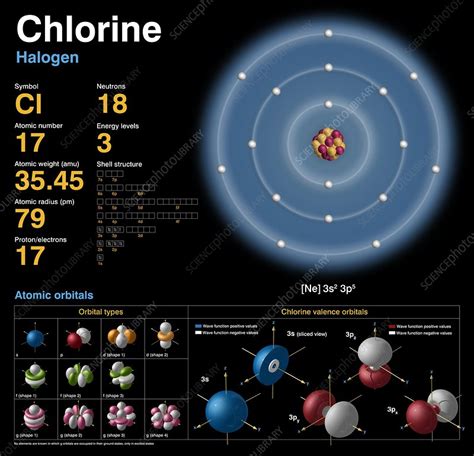
Table of Contents
How Many Atoms Are in Cl? Unpacking Avogadro's Number and Chemical Quantities
The question "How many atoms are in Cl?" is deceptively simple. It hinges on understanding the fundamental concepts of chemistry, particularly the mole and Avogadro's number. While "Cl" simply represents the element chlorine, the number of atoms depends on the context: are we talking about a single chlorine atom, a molecule of chlorine gas (Cl₂), or a specific amount of chlorine, such as a gram or a mole? Let's dive deep into this seemingly straightforward question and explore the fascinating world of atomic quantities.
Understanding the Basics: Atoms, Molecules, and Moles
Before tackling the core question, let's establish a strong foundation.
-
Atoms: Atoms are the fundamental building blocks of matter. Each element, like chlorine (Cl), is composed of atoms with a unique number of protons in their nucleus. Chlorine atoms have 17 protons.
-
Molecules: Molecules are formed when two or more atoms chemically bond together. Chlorine, in its natural state, exists as a diatomic molecule, meaning two chlorine atoms are bonded together (Cl₂). This is crucial when calculating the number of atoms.
-
Moles: A mole (mol) is a unit of measurement in chemistry that represents a specific number of particles, whether atoms, molecules, ions, or other entities. This number is Avogadro's number, approximately 6.022 x 10²³. One mole of any substance contains Avogadro's number of particles. This is analogous to a dozen (12) – a dozen eggs contains 12 eggs, just as a mole of chlorine atoms contains 6.022 x 10²³ chlorine atoms.
Calculating Atoms in Different Chlorine Scenarios
Now, let's address the variations of the question:
1. How many atoms are in a single chlorine atom (Cl)?
This one's straightforward: one. A single chlorine atom contains only one chlorine atom.
2. How many atoms are in a chlorine molecule (Cl₂)?
A chlorine molecule (Cl₂) consists of two chlorine atoms bonded together. Therefore, one molecule of chlorine contains two chlorine atoms.
3. How many atoms are in one mole of chlorine atoms (Cl)?
One mole of any substance contains Avogadro's number of particles. Therefore, one mole of chlorine atoms (Cl) contains 6.022 x 10²³ chlorine atoms.
4. How many atoms are in one mole of chlorine molecules (Cl₂)?
This is where it gets slightly more complex. One mole of chlorine molecules (Cl₂) contains Avogadro's number (6.022 x 10²³) of molecules. Since each molecule has two chlorine atoms, the total number of chlorine atoms is:
(6.022 x 10²³ molecules/mol) * (2 atoms/molecule) = 1.204 x 10²⁴ atoms
Therefore, one mole of chlorine gas (Cl₂) contains 1.204 x 10²⁴ chlorine atoms.
5. How many atoms are in a given mass of chlorine?
To determine the number of atoms in a specific mass of chlorine, we need to use the molar mass of chlorine. The molar mass of chlorine is approximately 35.45 grams per mole (g/mol) for chlorine atoms (Cl) and approximately 70.90 g/mol for chlorine molecules (Cl₂).
Let's say we have 70.90 grams of chlorine gas (Cl₂). We can use the following steps:
-
Convert grams to moles: Divide the mass (70.90 g) by the molar mass (70.90 g/mol). This gives us 1 mole of Cl₂.
-
Calculate the number of molecules: One mole of Cl₂ contains Avogadro's number (6.022 x 10²³) of molecules.
-
Calculate the number of atoms: Since each molecule has two chlorine atoms, multiply the number of molecules by 2.
This results in the same answer as before: 1.204 x 10²⁴ atoms.
If we had, for example, 35.45 grams of chlorine atoms (a hypothetical scenario since chlorine exists as a diatomic molecule), we'd have 1 mole, containing 6.022 x 10²³ atoms.
The Significance of Avogadro's Number
Avogadro's number is a cornerstone of chemistry, bridging the microscopic world of atoms and molecules to the macroscopic world of measurable quantities. It allows chemists to work with incredibly large numbers of atoms and molecules in a manageable way. Without this constant, many chemical calculations would be practically impossible.
Beyond Chlorine: Applying the Concepts to Other Elements and Compounds
The principles discussed here apply to all elements and compounds. To calculate the number of atoms in any substance, you need to consider:
- The chemical formula: This indicates the types and numbers of atoms in a molecule.
- The molar mass: This relates the mass of a substance to the number of moles.
- Avogadro's number: This is the conversion factor between moles and the number of particles.
Practical Applications and Real-World Examples
Understanding the relationship between atoms, moles, and Avogadro's number is vital in various fields, including:
- Industrial Chemistry: Determining the amount of reactants needed for chemical reactions in manufacturing processes.
- Pharmaceutical Science: Calculating dosages and determining the purity of drugs.
- Environmental Science: Analyzing the concentrations of pollutants and studying chemical reactions in the environment.
- Material Science: Designing and synthesizing new materials with specific properties.
Conclusion: Mastering Atomic Quantities
The seemingly simple question "How many atoms are in Cl?" opens up a rich understanding of fundamental chemical concepts. By grasping the roles of atoms, molecules, moles, and Avogadro's number, we can accurately calculate the number of atoms in various forms and quantities of chlorine and other substances. This knowledge is crucial for anyone working with chemicals, from industrial chemists to aspiring scientists. The ability to seamlessly transition between macroscopic measurements and the microscopic world of atoms is a hallmark of chemical proficiency. This understanding allows for accurate calculations, efficient processes, and the ability to predict and manipulate the behavior of matter at a fundamental level. As we've seen, the answer varies significantly depending on the form of chlorine being considered, highlighting the importance of precise chemical terminology and a solid understanding of fundamental concepts.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Monotheistic Religion
Mar 21, 2025
-
What Is 6 10 As A Percent
Mar 21, 2025
-
Convert Mixed Number To Decimal Calculator
Mar 21, 2025
-
Sugar Dissolves In Water Physical Or Chemical Change
Mar 21, 2025
-
What Is A Positive Ion Called
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
