How Many A Columns Are On The Periodic Table
Juapaving
Mar 13, 2025 · 6 min read
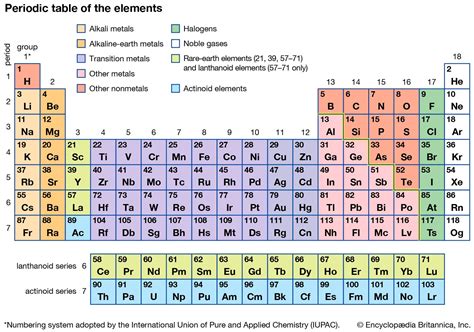
Table of Contents
How Many Columns Are on the Periodic Table? Exploring the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes known chemical elements in a structured grid, revealing patterns and relationships between their properties. A frequent question, especially for students new to chemistry, revolves around the number of columns, or groups, in this vital chart. While a simple answer might seem straightforward, a deeper exploration reveals a more nuanced understanding of the table's structure and the significance of its organization.
Understanding the Structure: Columns and Groups
The periodic table isn't just a random arrangement of elements. Its organization reflects the underlying electronic structure of atoms, specifically the number of electrons in their outermost shell, known as valence electrons. These valence electrons largely dictate an element's chemical behavior and reactivity. The columns, also referred to as groups or families, represent elements with similar valence electron configurations. This similarity leads to predictable patterns in their chemical properties.
So, how many columns are there? The answer depends on how you count them. A simplistic count would lead to 18 columns. However, a deeper dive reveals a more intricate organization.
The 18-Column View: The Standard Periodic Table
The most commonly presented periodic table displays 18 columns. This representation is often used in introductory chemistry courses and textbooks. These 18 columns are numbered from 1 to 18, using the IUPAC (International Union of Pure and Applied Chemistry) system, a globally accepted standard for chemical nomenclature. This numbering system simplifies discussions and avoids ambiguity caused by older numbering conventions.
This 18-column table effectively groups elements with similar chemical properties. For example:
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron (e.g., Lithium, Sodium, Potassium).
- Group 18 (Noble Gases): Inert gases with a full valence shell, making them extremely unreactive (e.g., Helium, Neon, Argon).
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons (e.g., Fluorine, Chlorine, Bromine).
These are just a few examples of the clear trends and similarities found within the groups of the 18-column periodic table.
Beyond the 18: Exploring Alternative Representations
While the 18-column periodic table is prevalent, variations exist, offering different perspectives on the organization of elements. These alternative representations highlight certain aspects of chemical behavior and electronic structure more clearly.
The Traditional A and B Group System: A Historical Perspective
Older periodic tables often employed an A and B group system. This system divided the groups into main groups (A groups) and transition groups (B groups). This system, while historical, can be confusing, especially for beginners, as it doesn’t align with the modern IUPAC numbering. Understanding this historical context is helpful for interpreting older literature but should be replaced by the modern 18-column system for clarity and consistency.
The Extended Periodic Table: Predicting Future Elements
The periodic table isn't static; it's constantly evolving. Scientists are actively synthesizing new, superheavy elements, expanding the table beyond its currently known limits. These hypothetical elements are often included in extended periodic tables, extending the number of columns beyond 18, reflecting theoretical predictions of their electronic configurations and chemical properties. These predictions are based on complex quantum mechanical calculations. These extended periodic tables provide a framework for discussing and researching the potential properties of these yet-to-be-fully characterized elements. The predictions remain theoretical until experimentally verified.
The Significance of the Columnar Organization
The organization of elements into columns isn't merely a visual arrangement; it's a powerful tool for understanding chemistry. The consistent patterns observed within groups allow chemists to:
- Predict chemical properties: Knowing an element's group allows for reasonable predictions about its reactivity, bonding behavior, and other chemical properties.
- Design new materials: Understanding the relationships between elements facilitates the design of new materials with specific properties. For instance, knowing the properties of elements in a specific group can help in developing new alloys or semiconductors.
- Understand chemical reactions: The periodic table provides a framework for understanding the mechanisms and driving forces behind chemical reactions. It helps to visualize the electron transfer and sharing that occurs during chemical processes.
- Develop chemical theories: The table itself is a product of underlying theories about atomic structure and chemical bonding. Its arrangement reflects and reinforces our understanding of the fundamental principles of chemistry.
The Importance of Valence Electrons in Columnar Organization
The core principle underpinning the columnar structure of the periodic table is the number of valence electrons. These outermost electrons are directly involved in chemical bonding, determining an element's reactivity. Elements within the same group possess the same number of valence electrons, leading to similar chemical behavior.
- Group 1 (Alkali Metals): One valence electron, readily lost in chemical reactions.
- Group 17 (Halogens): Seven valence electrons, readily gained in chemical reactions.
- Group 18 (Noble Gases): Eight valence electrons (except Helium with two), leading to their inert nature.
The similarity in valence electron configurations is the unifying factor that dictates the similar chemical behavior observed within each column.
Beyond Simple Columns: Subshells and Electron Configurations
While the 18-column structure provides a convenient overview, a more detailed understanding requires considering the subshells within the electron configurations of atoms. The filling of these subshells (s, p, d, f) influences the electronic structure and, consequently, the chemical properties of the elements.
- s-block elements: Groups 1 and 2, filling the s subshell.
- p-block elements: Groups 13-18, filling the p subshell.
- d-block elements: Transition metals, filling the d subshell.
- f-block elements: Lanthanides and actinides, filling the f subshell.
This more nuanced understanding reveals the subtle differences even within the groups, adding depth to our comprehension of elemental properties. The 18-column representation simplifies this complexity for educational purposes, but the underlying electron configurations provide a more complete picture.
Conclusion: A Dynamic and Ever-Evolving System
The question "How many columns are on the periodic table?" doesn't have a single definitive answer. While the most common representation shows 18 columns, considering historical conventions and the possibility of future discoveries complicates this seemingly simple query.
The periodic table's beauty lies in its ability to organize complex information in a way that reveals patterns and relationships, providing a fundamental framework for understanding chemistry. Its 18-column structure, while a simplification, remains a powerful tool for predicting and understanding the chemical behavior of elements. Its continual evolution, driven by scientific discoveries and advancements in our understanding of atomic structure, ensures its enduring relevance and importance in the chemical sciences. The 18 columns serve as a foundation for deeper explorations into the rich and diverse world of chemical elements and their interactions.
Latest Posts
Latest Posts
-
How Many Stereoisomers Are Possible For
May 09, 2025
-
14 Rounded To The Nearest Tenth
May 09, 2025
-
What Is The Si Unit For Distance
May 09, 2025
-
How Many Lines Of Symmetry Does H Have
May 09, 2025
-
18 Inches Equals How Many Feet
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many A Columns Are On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.