How Do You Separate A Heterogeneous Mixture
Juapaving
Apr 01, 2025 · 6 min read
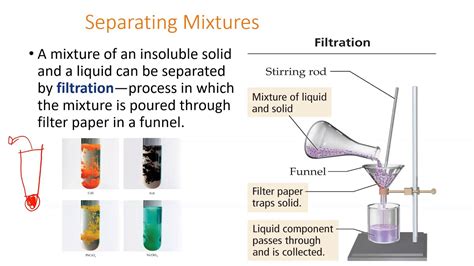
Table of Contents
How Do You Separate a Heterogeneous Mixture? A Comprehensive Guide
Heterogeneous mixtures are everywhere – from the salad on your plate to the rocks in your garden. Understanding how to separate these mixtures is crucial in various fields, from chemistry and geology to food science and environmental engineering. This comprehensive guide will explore the various techniques used to separate heterogeneous mixtures, detailing their principles, applications, and limitations.
What is a Heterogeneous Mixture?
Before delving into separation techniques, let's define our subject. A heterogeneous mixture is a combination of two or more substances where the different components are visibly distinguishable. Unlike homogeneous mixtures (like saltwater), where the components are uniformly distributed at a molecular level, heterogeneous mixtures have distinct phases or regions with different compositions. Think of a pizza: you can clearly see the separate components like cheese, pepperoni, sauce, and crust.
Common Methods for Separating Heterogeneous Mixtures
Several methods exist for separating heterogeneous mixtures, each best suited to specific types of mixtures and the properties of their components. The choice of technique depends on factors like the size and density of the particles, their solubility, and their magnetic properties.
1. Handpicking
This is the simplest method and involves manually separating components based on their visible differences. It's effective for separating larger, easily identifiable components from a mixture.
- Principle: Relies on visual identification and manual dexterity.
- Applications: Separating pebbles from sand, picking out unwanted materials from grains, sorting fruits and vegetables.
- Limitations: Inefficient for large quantities, unsuitable for small or similar-looking components, prone to human error.
2. Sieving
Sieving utilizes a sieve or mesh with specific pore sizes to separate components based on their particle size. Larger particles are retained, while smaller ones pass through.
- Principle: Exploits differences in particle size.
- Applications: Separating sand from gravel, separating flour from impurities, sifting soil to remove stones.
- Limitations: Not effective for separating particles of similar sizes, requires multiple sieves for precise separation.
3. Sedimentation and Decantation
This technique relies on the difference in density between the components. The denser component settles at the bottom (sedimentation), allowing the lighter component to be carefully poured off (decantation).
- Principle: Utilizes gravity and density differences.
- Applications: Separating sand from water, removing sediments from a liquid, clarifying muddy water.
- Limitations: Slow process for fine particles, complete separation may not be achieved, ineffective for components with similar densities.
4. Filtration
Filtration employs a porous material (like filter paper) to separate solid particles from a liquid or gaseous mixture. The liquid or gas passes through the filter, while the solid particles are trapped.
- Principle: Uses a porous barrier to separate components based on particle size.
- Applications: Separating sand from water, filtering coffee, purifying water, removing dust from air.
- Limitations: May be slow for large volumes, filter may become clogged, not suitable for separating very fine particles or dissolved substances.
5. Evaporation
Evaporation involves heating a liquid mixture to vaporize the liquid component, leaving behind the solid solute.
- Principle: Uses the difference in boiling points of the components.
- Applications: Obtaining salt from seawater, drying clothes, concentrating solutions.
- Limitations: Not suitable for separating components with similar boiling points, can be energy-intensive, some components might decompose upon heating.
6. Distillation
Distillation is a more sophisticated technique used to separate liquids with different boiling points. The mixture is heated, and the vapor of the more volatile component is collected and condensed separately.
- Principle: Uses differences in boiling points to separate liquids.
- Applications: Producing purified water, separating alcohol from water, refining petroleum.
- Limitations: Requires specialized equipment, energy-intensive, may not be effective for components with very similar boiling points.
7. Centrifugation
Centrifugation uses centrifugal force to separate components with different densities. The mixture is spun rapidly, forcing the denser components to settle at the bottom.
- Principle: Uses centrifugal force to accelerate sedimentation.
- Applications: Separating blood components, separating cream from milk, clarifying suspensions.
- Limitations: Requires specialized equipment, may not be effective for very fine particles or components with similar densities.
8. Magnetic Separation
This method exploits the magnetic properties of components. A magnet is used to attract and separate magnetic materials from a mixture.
- Principle: Uses the magnetic properties of certain materials.
- Applications: Separating iron filings from sand, removing magnetic impurities from ores.
- Limitations: Only effective for separating magnetic materials, not suitable for non-magnetic components.
9. Chromatography
Chromatography is a powerful technique used to separate components based on their differing affinities for a stationary phase and a mobile phase. The mixture is passed through a column or a planar surface, and components with different affinities will travel at different speeds, leading to separation. There are many forms of chromatography, including paper chromatography, thin-layer chromatography (TLC), and column chromatography.
- Principle: Exploits differential adsorption or partitioning of components between two phases.
- Applications: Separating pigments in ink, analyzing mixtures of chemicals, purifying substances.
- Limitations: Can be complex and time-consuming, requires specialized equipment for certain types of chromatography.
10. Sublimation
Sublimation involves the direct transition of a solid to a gas, bypassing the liquid phase. This method is useful for separating a solid that sublimes from other non-sublimable components.
- Principle: Uses the difference in sublimation properties of components.
- Applications: Separating iodine from sand, purifying naphthalene.
- Limitations: Only effective for substances that sublime, requires controlled temperature and pressure.
Choosing the Right Separation Technique
The selection of the appropriate separation technique depends heavily on the characteristics of the heterogeneous mixture. Consider these factors:
- Size and shape of particles: Handpicking, sieving, and filtration are suitable for mixtures with visibly different particle sizes.
- Density differences: Sedimentation, decantation, and centrifugation are effective when components have significantly different densities.
- Solubility: Evaporation, distillation, and chromatography are useful when components have different solubilities.
- Boiling points: Distillation is ideal for separating liquids with different boiling points.
- Magnetic properties: Magnetic separation is specifically useful for magnetic components.
- Quantity of the mixture: Handpicking is suitable for small quantities, while industrial-scale separation techniques are needed for large volumes.
Advanced Separation Techniques
Beyond the basic methods, several advanced techniques exist for separating complex heterogeneous mixtures. These often involve sophisticated equipment and principles. Examples include:
- Electrostatic separation: Uses electrostatic charges to separate particles based on their electrical properties.
- Froth flotation: Uses air bubbles to separate hydrophobic materials from hydrophilic ones.
- Crystallization: Separates components based on their differences in solubility at different temperatures.
Conclusion
Separating heterogeneous mixtures is a fundamental process with applications across numerous scientific disciplines and industries. Understanding the principles behind various separation techniques allows for the efficient and effective isolation of individual components from complex mixtures. The optimal method depends on the specific properties of the mixture and the desired outcome. By carefully considering the characteristics of the mixture and selecting the appropriate technique, the efficient separation of components becomes achievable. This knowledge empowers advancements in fields ranging from material science and pharmaceuticals to environmental remediation and food processing.
Latest Posts
Latest Posts
-
What Is 30 Percent Of 120
Apr 02, 2025
-
Difference Between Violet And Purple Color
Apr 02, 2025
-
How Many Mm Is 3 Cm
Apr 02, 2025
-
A Red Blood Cell Placed In A Hypertonic Medium Will
Apr 02, 2025
-
What Is The Conjugate Acid Of Nh3
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Do You Separate A Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
