How Are Mixtures And Solutions Different
Juapaving
Mar 14, 2025 · 5 min read
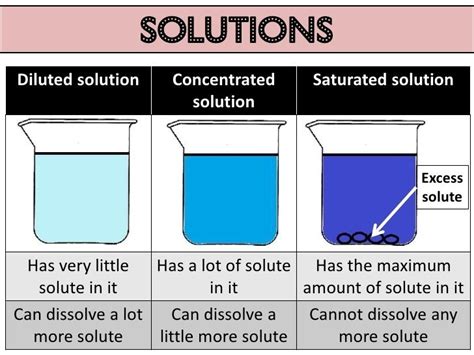
Table of Contents
How Are Mixtures and Solutions Different? A Deep Dive into Chemistry
Understanding the fundamental differences between mixtures and solutions is crucial for anyone studying chemistry, or simply curious about the world around them. While both involve combining two or more substances, the way these substances interact and the resulting properties differ significantly. This article will explore these differences in detail, explaining the key characteristics of mixtures and solutions, providing real-world examples, and clarifying common misconceptions.
What is a Mixture?
A mixture is a substance comprising two or more components not chemically bonded. A key characteristic is that the components retain their individual chemical properties. This means you can physically separate the components of a mixture using techniques like filtration, distillation, evaporation, or chromatography. The proportions of each component in a mixture can vary.
Types of Mixtures:
Mixtures are broadly classified into two categories:
1. Heterogeneous Mixtures: These mixtures have a non-uniform composition. Different regions of the mixture have different properties and compositions. You can easily distinguish the individual components with the naked eye or under a microscope.
- Examples: Sand and water, oil and water, salad dressing, granite rock, concrete.
2. Homogeneous Mixtures: These mixtures have a uniform composition throughout. The components are evenly distributed at a microscopic level, making it difficult to visually distinguish them. They appear as a single phase.
- Examples: Saltwater, air (a mixture of gases), sugar dissolved in water (before we delve into solutions, this is a good example of a homogeneous mixture).
Key Characteristics of Mixtures:
- Variable Composition: The ratio of components can vary.
- Retention of Individual Properties: Components retain their original chemical properties.
- Easily Separable: Components can be separated using physical methods.
- No Chemical Reaction: There is no chemical reaction between the components.
What is a Solution?
A solution is a special type of homogeneous mixture where one substance, the solute, is dissolved in another substance, the solvent. The resulting solution is a single, homogeneous phase, meaning it has a uniform composition throughout. The solute particles are distributed uniformly at the molecular or ionic level within the solvent.
Understanding Solutes and Solvents:
- Solute: The substance that dissolves in the solvent. It is usually present in a smaller amount than the solvent.
- Solvent: The substance that dissolves the solute. It is usually present in a larger amount. Water is the most common solvent.
Types of Solutions:
Solutions can be formed from different combinations of states of matter:
- Solid in Liquid: Saltwater (salt is the solute, water is the solvent).
- Liquid in Liquid: Alcohol in water.
- Gas in Liquid: Carbonated water (carbon dioxide is the solute, water is the solvent).
- Gas in Gas: Air (various gases dissolved in each other).
- Solid in Solid: Alloys like brass (zinc and copper).
Key Characteristics of Solutions:
- Homogeneous: Uniform composition throughout.
- Particle Size: Solute particles are extremely small (ions or molecules).
- Transparent: Solutions are usually transparent (though the color may vary depending on the solute).
- Difficult to Separate: Separating the solute from the solvent often requires more complex methods than physical separation, such as distillation or crystallization.
- No Settling: Solute particles do not settle out over time.
The Crucial Differences Between Mixtures and Solutions:
The table below summarizes the key distinctions between mixtures and solutions:
| Feature | Mixture | Solution |
|---|---|---|
| Composition | Variable, can be heterogeneous or homogeneous | Always homogeneous |
| Particle Size | Can vary widely | Extremely small (ions or molecules) |
| Separation | Easily separated by physical methods | Difficult to separate; requires specialized techniques |
| Properties | Components retain individual properties | Components' properties may change |
| Uniformity | May or may not be uniform | Always uniform |
| Appearance | Can be opaque, translucent, or transparent | Usually transparent |
| Examples | Sand and water, oil and water, air | Saltwater, sugar water, air (considered a solution of gases) |
Examples in Everyday Life:
Understanding the difference between mixtures and solutions is relevant to many aspects of our daily lives:
- Cooking: Making a cake batter involves mixing various ingredients (flour, sugar, eggs, etc.) to form a heterogeneous mixture. Dissolving sugar in coffee creates a solution.
- Cleaning: Cleaning solutions are often solutions of various chemicals in water.
- Medicine: Many medications are solutions or suspensions (a type of mixture).
- Environmental Science: Understanding the composition of air (a solution of gases) and water (which can contain various dissolved substances) is essential for environmental monitoring and protection.
- Industrial Processes: Many industrial processes rely on creating and manipulating solutions, for example, in electroplating or chemical synthesis.
Advanced Concepts and Misconceptions:
1. Colloids: Colloids represent a gray area between mixtures and solutions. They are homogeneous mixtures but contain larger particles than solutions (1-1000 nm). These particles don't settle out like in a heterogeneous mixture but can scatter light (Tyndall effect), distinguishing them from true solutions. Milk and fog are examples of colloids.
2. Suspensions: Suspensions are heterogeneous mixtures where larger particles are dispersed in a liquid or gas. These particles will settle out over time if left undisturbed. Muddy water is a classic example.
3. Concentration: The concentration of a solution refers to the amount of solute dissolved in a given amount of solvent or solution. This can be expressed in various ways, such as molarity, molality, or percent concentration.
4. Solubility: Solubility refers to the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure. Factors such as temperature and pressure significantly impact solubility.
Conclusion:
While mixtures and solutions both involve combining substances, the crucial difference lies in the size of the particles and the degree of interaction between them. Solutions are homogeneous mixtures characterized by uniformly dissolved particles at the molecular level, while mixtures can be homogeneous or heterogeneous with larger, less uniformly distributed particles. Understanding these distinctions is critical for various scientific and everyday applications, allowing for better comprehension of chemical processes and interactions within our world. This deep dive provides a solid foundation for further exploration of these fundamental concepts in chemistry. Further research into specific types of mixtures, solutions, and their applications will enhance your understanding and appreciation of this fascinating area of science.
Latest Posts
Latest Posts
-
What Is The Mass Number Of Calcium
Mar 14, 2025
-
Why Earth Is Called Blue Planet
Mar 14, 2025
-
What Is The Prime Factorization For 22
Mar 14, 2025
-
What Is A Multiple Of 13
Mar 14, 2025
-
What Is The Percentage Of 2 8
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Are Mixtures And Solutions Different . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
