What Is The Mass Number Of Calcium
Juapaving
Mar 14, 2025 · 5 min read
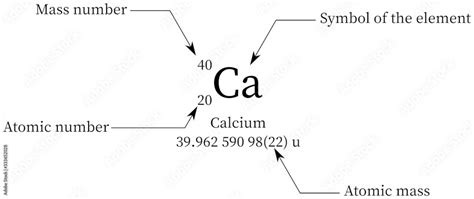
Table of Contents
What is the Mass Number of Calcium? Understanding Atomic Mass and Isotopes
Calcium, a vital element for life, plays a crucial role in various biological processes. Understanding its atomic structure, particularly its mass number, is essential for grasping its chemical behavior and biological significance. This article delves into the intricacies of calcium's mass number, exploring the concept of isotopes and their impact on the element's overall properties. We'll also discuss how mass number relates to atomic weight and its significance in various scientific fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the mass number of calcium, let's refresh our understanding of fundamental atomic concepts. Every atom comprises three subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and its identity. For calcium, the atomic number is 20, meaning every calcium atom has 20 protons.
-
Neutrons: Neutral particles also located in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the existence of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom.
What is Mass Number?
The mass number of an atom is the total number of protons and neutrons in its nucleus. It's a whole number representing the atom's total mass, expressed in atomic mass units (amu). Since protons and neutrons have approximately equal mass (1 amu each), the mass number provides a good approximation of the atom's mass. It's often represented as a superscript to the left of the element's symbol (e.g., ²⁰⁶Pb indicates lead with a mass number of 206).
Isotopes: The Key to Understanding Calcium's Mass Number
The concept of isotopes is crucial for understanding the mass number of calcium. Isotopes are atoms of the same element (same number of protons) but with varying numbers of neutrons. This means they have the same atomic number but different mass numbers.
Calcium has several naturally occurring isotopes. Each isotope is identified by its mass number. For example:
-
⁴⁰Ca: This is the most abundant isotope of calcium, accounting for approximately 97% of naturally occurring calcium. It has 20 protons and 20 neutrons (20 + 20 = 40).
-
⁴²Ca: This isotope has 20 protons and 22 neutrons (20 + 22 = 42).
-
⁴³Ca: This isotope contains 20 protons and 23 neutrons (20 + 23 = 43).
-
⁴⁴Ca: This isotope contains 20 protons and 24 neutrons (20 + 24 = 44).
-
⁴⁶Ca: This isotope contains 20 protons and 26 neutrons (20 + 26 = 46).
-
⁴⁸Ca: This is the least abundant stable isotope, and it possesses 20 protons and 28 neutrons (20 + 28 = 48).
There are also radioactive isotopes of calcium, which are unstable and decay over time. These isotopes are not naturally abundant and are often produced artificially.
Calcium's Average Atomic Mass: A Weighted Average of Isotopes
The periodic table lists the atomic weight (or average atomic mass) of calcium, not a single mass number. This is because the atomic weight is a weighted average of the mass numbers of all naturally occurring isotopes, considering their relative abundances. Since ⁴⁰Ca is the most prevalent isotope, it significantly influences the overall average atomic mass.
The atomic weight of calcium is approximately 40.078 amu. This value reflects the combined contribution of all the stable calcium isotopes and their relative abundances in nature.
Significance of Calcium's Mass Number and Isotopes in Biology and Science
The different isotopes of calcium, while chemically similar, can have subtle differences in their physical properties. These differences, though small, can be significant in various scientific applications. For example:
-
Biological Processes: The most abundant isotope, ⁴⁰Ca, is crucial for various biological functions, including bone formation, muscle contraction, nerve impulse transmission, and blood clotting. The slightly different masses of other isotopes might influence the rate of these processes, although the effects are generally minor compared to the dominant role of ⁴⁰Ca.
-
Radioactive Isotopes as Tracers: Radioactive calcium isotopes, such as ⁴⁵Ca, are used as tracers in biological and medical research. Their radioactive decay allows scientists to track the movement and distribution of calcium within living organisms. This is invaluable in studying calcium metabolism and related diseases.
-
Geological Dating: The ratios of different calcium isotopes in geological samples can be used to determine the age of rocks and minerals. This technique is particularly useful for dating very old geological formations.
-
Nuclear Physics: Calcium isotopes, particularly those with a large neutron excess, are subjects of interest in nuclear physics research. Studying their properties can provide insights into the structure of atomic nuclei and the forces that bind them.
-
Industrial Applications: While less prevalent, specific isotopes might have specialized applications in industrial processes where precise mass or radioactive properties are needed.
Beyond the Mass Number: Other Important Properties of Calcium
While the mass number is a fundamental aspect of calcium's atomic structure, other properties are crucial to its behavior and applications:
-
Electron Configuration: Calcium's electron configuration ([Ar]4s²) determines its chemical reactivity and tendency to form ions. Its two valence electrons are readily lost, leading to the formation of a Ca²⁺ ion. This property is essential for its role in many biological processes and chemical reactions.
-
Chemical Reactivity: Calcium is a relatively reactive alkaline earth metal. Its reactivity is linked to its electron configuration and its tendency to lose electrons. This reactivity dictates its participation in various chemical reactions and its interactions with other elements.
-
Physical Properties: Calcium is a silvery-white metal with relatively low density and melting point. These properties influence its industrial applications and its handling.
Conclusion: The Mass Number of Calcium in a Broader Context
The mass number of calcium, although a single number for each isotope, is a fundamental aspect of understanding its atomic structure, chemical behavior, and biological significance. The existence of multiple isotopes, each with its own mass number, affects the average atomic mass listed on the periodic table and plays a role in various scientific and technological applications, from biological tracing to geological dating and nuclear research. Understanding the interplay between mass number, isotopes, and other atomic properties provides a comprehensive understanding of calcium's importance in our world. Further research continues to reveal the intricacies of calcium's isotopes and their influence on diverse fields.
Latest Posts
Latest Posts
-
Is Slime A Non Newtonian Fluid
Mar 14, 2025
-
What Type Of Triangle Has Two Equal Sides
Mar 14, 2025
-
What Is The Function Of Areolar Tissue
Mar 14, 2025
-
Nouns That Start With An A
Mar 14, 2025
-
What Is 8 10 As A Percentage
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Number Of Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
