Fundamental Building Blocks Of All Matter
Juapaving
Mar 26, 2025 · 8 min read
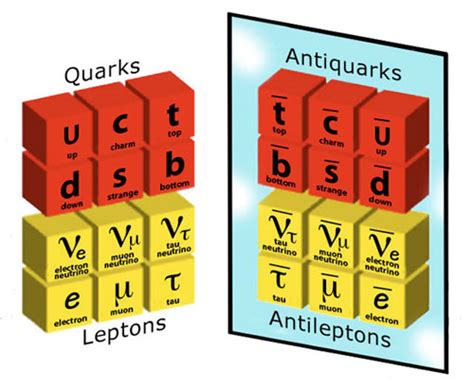
Table of Contents
Fundamental Building Blocks of All Matter: A Deep Dive into Atoms, Elements, and Beyond
The universe, in all its breathtaking complexity and vastness, is fundamentally constructed from a surprisingly small number of basic building blocks. Understanding these fundamental components is key to unlocking the secrets of matter, from the smallest subatomic particle to the largest galaxies. This article delves into the intricate world of atoms, elements, and the forces that govern their interactions, providing a comprehensive overview of the fundamental building blocks of all matter.
Atoms: The Indivisible Units?
For centuries, philosophers and scientists debated the nature of matter. The Greek philosopher Democritus proposed the concept of atomos, meaning "indivisible," suggesting that matter was composed of tiny, indestructible particles. While his idea was insightful, it lacked experimental evidence. It wasn't until the late 19th and early 20th centuries that the atomic theory gained scientific credence.
The Atomic Model Evolves
Early models of the atom were relatively simple. John Dalton's atomic theory, proposed in the early 1800s, posited that atoms were solid, indivisible spheres. However, subsequent discoveries revealed a far more complex structure. J.J. Thomson's experiments with cathode rays led to the discovery of the electron, a negatively charged subatomic particle. This shattered the idea of the atom as an indivisible unit, leading to the "plum pudding" model, where negatively charged electrons were embedded within a positively charged sphere.
Ernest Rutherford's gold foil experiment dramatically altered our understanding. By bombarding a thin gold foil with alpha particles, Rutherford observed that a significant number of particles were deflected at large angles, suggesting the presence of a dense, positively charged nucleus at the atom's center. This led to the development of the nuclear model, where electrons orbit a small, dense nucleus containing positively charged protons.
The Quantum Leap: Unveiling the Subatomic World
The nuclear model, while a significant improvement, still couldn't fully explain the behavior of atoms. The development of quantum mechanics revolutionized our understanding, revealing a far more intricate and probabilistic subatomic world. Niels Bohr's model incorporated quantum theory, suggesting that electrons orbit the nucleus in specific energy levels or shells. Electrons can jump between these energy levels by absorbing or emitting photons of light.
This quantum mechanical model paved the way for a more accurate description of atomic structure. We now know that the behavior of electrons is governed by probability, and their location can only be described statistically. The electron cloud model depicts electrons as existing within regions of space around the nucleus, with the probability of finding an electron at a particular location represented by the density of the electron cloud.
Subatomic Particles: Delving Deeper
The atom is far from the ultimate building block of matter. It is composed of even smaller particles:
Protons: The Positive Charge Carriers
Protons, located within the nucleus, carry a positive charge equal in magnitude to the electron's negative charge. The number of protons in an atom's nucleus determines its atomic number and defines the element.
Neutrons: The Neutral Partners
Neutrons, also residing in the nucleus, are electrically neutral particles. They contribute to the atom's mass but not its charge. The number of neutrons in an atom's nucleus can vary, leading to different isotopes of the same element. Isotopes have the same number of protons but different numbers of neutrons.
Electrons: The Orbiting Negatives
Electrons are negatively charged particles that orbit the nucleus. They are much smaller and lighter than protons and neutrons. The arrangement of electrons in an atom's electron shells determines its chemical properties and how it interacts with other atoms.
Elements: The Building Blocks of Molecules
An element is a pure substance consisting of only one type of atom. Each element is uniquely identified by its atomic number, which represents the number of protons in its nucleus. The periodic table arranges elements based on their atomic number and recurring chemical properties.
The Periodic Table: A Systematic Organization
The periodic table is a powerful tool that organizes all known elements based on their atomic number, electron configuration, and recurring chemical properties. Elements with similar properties are grouped together in columns (groups or families), while rows (periods) represent elements with the same number of electron shells.
Properties of Elements
The properties of an element are determined by the number of protons and electrons it possesses. These properties include:
- Atomic mass: The average mass of an atom of an element, considering the relative abundance of its isotopes.
- Electronegativity: The tendency of an atom to attract electrons towards itself in a chemical bond.
- Ionization energy: The energy required to remove an electron from an atom.
- Reactivity: The tendency of an atom to react with other atoms to form chemical bonds.
Molecules and Compounds: The Emergence of Complexity
Atoms rarely exist in isolation. They tend to interact with each other, forming molecules and compounds.
Chemical Bonds: The Force of Attraction
Atoms are held together in molecules and compounds through chemical bonds. These bonds arise from the electrostatic forces between atoms. The primary types of chemical bonds include:
- Covalent bonds: These bonds form when atoms share electrons to achieve a stable electron configuration. Covalent bonds are typically strong and found in many organic molecules.
- Ionic bonds: These bonds form when one atom transfers an electron to another, creating oppositely charged ions that attract each other. Ionic bonds are prevalent in salts.
- Metallic bonds: These bonds occur in metals, where electrons are delocalized and shared amongst a "sea" of electrons. This accounts for the properties of metals, such as their conductivity and malleability.
Molecules: Groups of Atoms
A molecule is a group of two or more atoms bonded together. Molecules can be composed of atoms of the same element (e.g., O2, oxygen gas) or different elements (e.g., H2O, water).
Compounds: Mixtures of Elements
A compound is a substance composed of two or more different elements chemically bonded together in a fixed ratio. Compounds have unique properties that differ from the properties of their constituent elements. For example, water (H2O) is a liquid at room temperature, while hydrogen and oxygen are gases.
Beyond Atoms and Molecules: The Realm of Condensed Matter
The interactions between atoms and molecules lead to the formation of various states of matter: solids, liquids, and gases. But the story doesn't end there. Understanding the collective behavior of vast numbers of atoms and molecules opens up the fascinating world of condensed matter physics.
Solids: Ordered Structures
In solids, atoms or molecules are tightly packed together in a regular, ordered arrangement called a crystal lattice. The strong interatomic forces restrict the movement of particles, giving solids their rigid structure and fixed shape. Different types of solids exist, including crystalline solids (with a highly ordered structure), amorphous solids (with a disordered structure), and polymers (large molecules formed from repeating units).
Liquids: Flowing Matter
In liquids, atoms or molecules are less tightly packed than in solids, allowing them to move past one another. This gives liquids their ability to flow and take the shape of their container. The intermolecular forces in liquids are weaker than in solids, but still significant enough to keep the molecules relatively close together.
Gases: Independent Particles
In gases, atoms or molecules are widely separated and move independently of each other. The weak intermolecular forces allow gases to expand to fill their container and be easily compressed.
The Four Fundamental Forces: Governing Interactions
The interactions between the fundamental building blocks of matter are governed by four fundamental forces:
1. Strong Nuclear Force: Holding the Nucleus Together
The strong nuclear force is the strongest of the four fundamental forces. It acts within the atomic nucleus, binding protons and neutrons together despite the electrostatic repulsion between the positively charged protons. This force is short-range, acting only over distances comparable to the size of the nucleus.
2. Electromagnetic Force: Interactions Between Charges
The electromagnetic force governs the interactions between charged particles. It is responsible for the attraction between electrons and the nucleus, as well as the interactions between atoms and molecules. This force is long-range, meaning its influence extends over large distances, although it weakens with distance.
3. Weak Nuclear Force: Radioactive Decay
The weak nuclear force is responsible for radioactive decay, a process where unstable atomic nuclei transform into more stable ones. This force plays a crucial role in nuclear reactions and processes within stars. It's a short-range force, even shorter than the strong force.
4. Gravity: The Force of Attraction Between Masses
Gravity is the weakest of the four fundamental forces, but it is long-range and acts on all objects with mass. It is responsible for the formation of planets, stars, and galaxies. While it has a negligible effect at the atomic and molecular level, it dominates on larger scales, shaping the structure of the universe.
Conclusion: A Journey of Discovery Continues
Our understanding of the fundamental building blocks of matter has come a long way, from the ancient concept of atomos to the intricate quantum mechanical models of today. However, our journey of discovery continues. Scientists are continually pushing the boundaries of knowledge, exploring new particles and forces, and seeking a deeper understanding of the universe's fundamental structure. This ongoing exploration promises even more exciting revelations about the nature of reality and the building blocks that make up everything we see and experience.
Latest Posts
Latest Posts
-
Lcm Of 8 9 And 6
Mar 29, 2025
-
The Smallest Basic Unit Of Matter Is
Mar 29, 2025
-
Correctly Label The Following Parts Of The Digestive System
Mar 29, 2025
-
Five Letter Word Starts With Vi
Mar 29, 2025
-
Why Is Air A Homogeneous Mixture
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Fundamental Building Blocks Of All Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
