Freezing Temp Of Water In Kelvin
Juapaving
Mar 30, 2025 · 5 min read
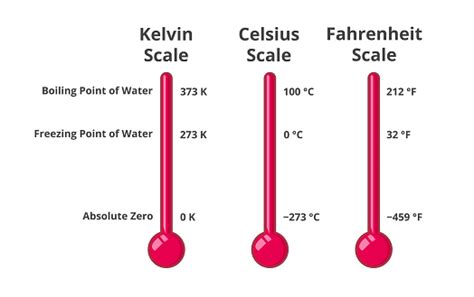
Table of Contents
The Freezing Point of Water in Kelvin: A Deep Dive
The freezing point of water is a fundamental concept in science, crucial for countless applications across various fields. While most people are familiar with 0° Celsius or 32° Fahrenheit, understanding this temperature in Kelvin is essential for many scientific and engineering purposes. This article will delve deep into the freezing point of water in Kelvin, exploring its significance, the underlying physics, and its practical implications. We'll also touch upon factors that can affect this seemingly simple phenomenon.
Understanding the Kelvin Scale
Before we dive into the freezing point of water in Kelvin, let's establish a firm understanding of the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales based on arbitrary reference points (the freezing and boiling points of water), the Kelvin scale is an absolute thermodynamic temperature scale. This means it starts at absolute zero, the theoretical point where all molecular motion ceases.
-
Absolute Zero: This theoretical point is 0 Kelvin (0 K), equivalent to -273.15° Celsius or -459.67° Fahrenheit. It's important to note that absolute zero is unattainable in practice, though scientists have come remarkably close.
-
Kelvin's Relationship to Celsius: The Kelvin scale is directly related to the Celsius scale. The size of one Kelvin degree is identical to one Celsius degree. To convert Celsius to Kelvin, you simply add 273.15. Therefore, 0°C is equivalent to 273.15 K.
-
Significance of the Absolute Scale: The absolute nature of the Kelvin scale is its most significant advantage. It simplifies many thermodynamic calculations and provides a consistent, universal standard for measuring temperature.
The Freezing Point of Water in Kelvin: 273.15 K
The freezing point of pure water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 273.15 Kelvin (K). This is a critical value in numerous scientific disciplines, including:
-
Chemistry: Many chemical reactions are temperature-dependent, and knowing the exact freezing point of water in Kelvin is crucial for precise experimental control and accurate results.
-
Physics: The behavior of water at and around its freezing point is essential for understanding concepts like phase transitions, latent heat, and thermal expansion.
-
Engineering: Engineering applications, such as designing cooling systems, require precise knowledge of the freezing point of water to prevent damage from freezing. This is particularly relevant in civil engineering (e.g., designing roads and bridges in cold climates) and mechanical engineering (e.g., designing efficient refrigeration systems).
-
Meteorology: Accurate temperature measurements in Kelvin are essential for meteorological modeling and weather forecasting, particularly in understanding the formation of ice and snow.
Factors Affecting the Freezing Point of Water
While 273.15 K is the standard freezing point, several factors can subtly influence the actual freezing point of water:
1. Pressure:
-
Pressure's Impact: Increased pressure slightly lowers the freezing point of water. This is an unusual property, unlike most substances where increased pressure raises the freezing point. This is because ice is less dense than liquid water.
-
Practical Implications: This effect is minimal under normal conditions but becomes significant at very high pressures, as encountered in deep-sea environments or in specialized high-pressure experiments.
2. Impurities:
-
Freezing Point Depression: Dissolving substances (solutes) in water lowers its freezing point. This phenomenon, known as freezing point depression, is directly proportional to the concentration of the solute. The more solute present, the lower the freezing temperature.
-
Examples: Saltwater, for instance, freezes at a lower temperature than pure water, which explains why salt is used to de-ice roads in winter. This principle is also vital in many industrial processes and applications.
3. Isotopes:
-
Isotopic Effects: The isotopic composition of water can also influence its freezing point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen freeze at slightly higher temperatures than water containing only the lighter isotopes.
-
Significance: This effect is relatively small but measurable and important in specialized research areas like isotope geochemistry and climate science.
The Importance of Precision in Measuring the Freezing Point
The precision with which the freezing point of water can be measured is crucial for various scientific and technological advancements. Highly accurate measurements require sophisticated equipment and meticulous procedures. Techniques such as differential scanning calorimetry (DSC) allow for extremely precise determination of phase transition temperatures, including the freezing point of water. These highly precise measurements are used for:
-
Calibration of Instruments: Accurate freezing point measurements are used to calibrate thermometers and other temperature-measuring instruments to ensure reliable measurements across various applications.
-
Fundamental Research: Precise measurements contribute to a deeper understanding of the fundamental physics and chemistry of water, furthering our knowledge of phase transitions, molecular interactions, and other related phenomena.
-
Material Science: The freezing point of water is crucial in material science research, particularly in the study of ice formation and the effects of freezing on different materials.
Applications of the Freezing Point of Water in Kelvin
The freezing point of water in Kelvin finds practical applications in a wide range of fields:
-
Cryopreservation: Freezing biological samples, such as cells, tissues, and organs, requires precise temperature control to prevent damage from ice crystal formation. Understanding the freezing point in Kelvin helps optimize cryopreservation protocols.
-
Food Science: The freezing point of water is critical in food preservation. Different foods freeze at slightly different temperatures due to their varying composition, and understanding these variations is crucial for optimizing food freezing processes.
-
Environmental Science: Accurate temperature measurements are crucial in environmental science, for studying the effects of temperature changes on ecosystems, including the role of ice and snow in climate change.
Conclusion: The Significance of 273.15 K
The freezing point of water at 273.15 K is not merely a numerical value; it's a fundamental constant that underpins numerous scientific and technological applications. Its precise measurement and understanding are essential for advancements in various disciplines. From cryopreservation to environmental science, the significance of this temperature cannot be overstated. This seemingly simple fact forms the basis of countless complex processes and discoveries, highlighting the profound impact of fundamental scientific knowledge. The ongoing research into the nuanced factors that influence the freezing point of water continues to refine our understanding of this crucial aspect of the natural world and fuels innovations in a multitude of fields. The ongoing refinement of measurement techniques and the exploration of its subtle variations in different conditions underscore the continuing importance of this seemingly straightforward, yet vitally significant, temperature.
Latest Posts
Latest Posts
-
Which Organelle Is Responsible For Photosynthesis
Apr 01, 2025
-
Which Is Larger Pound Or Kilogram
Apr 01, 2025
-
Are Histograms And Bar Graphs The Same
Apr 01, 2025
-
How Many Vowels Are There In English Language
Apr 01, 2025
-
Two Angles That Add Up To 90 Degrees
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Freezing Temp Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
