Freezing Point For Water In Kelvin
Juapaving
Mar 21, 2025 · 5 min read
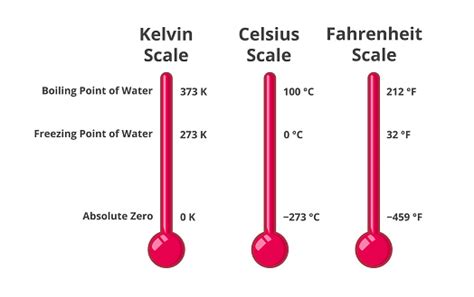
Table of Contents
Freezing Point of Water in Kelvin: A Deep Dive into Temperature Scales and Applications
The freezing point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. Understanding this point, particularly when expressed in Kelvin, unlocks a deeper appreciation for thermodynamics, phase transitions, and the fundamental properties of matter. This comprehensive article will explore the freezing point of water in Kelvin, delving into its significance, the intricacies of the Kelvin scale, and diverse applications across various fields.
Understanding Temperature Scales
Before diving into the specifics of water's freezing point in Kelvin, let's establish a firm understanding of different temperature scales. Three primary scales are commonly used:
-
Celsius (°C): Based on the freezing and boiling points of water at standard atmospheric pressure, with 0°C representing freezing and 100°C representing boiling. This scale is widely used globally for everyday applications.
-
Fahrenheit (°F): Primarily used in the United States, this scale defines the freezing point of water at 32°F and the boiling point at 212°F. A more complex scale with a less intuitive relationship to the properties of water.
-
Kelvin (K): This absolute temperature scale is the cornerstone of thermodynamic calculations. It starts at absolute zero, theoretically the lowest possible temperature where all molecular motion ceases. The Kelvin scale increments are identical to Celsius, but it offsets the zero point.
The Significance of Absolute Zero
Absolute zero (0 K or -273.15°C) is a critical concept in physics and thermodynamics. It marks the point where all matter possesses minimal energy. While theoretically unattainable, scientists have achieved temperatures incredibly close to absolute zero, revealing fascinating quantum phenomena. The Kelvin scale's foundation on absolute zero provides a consistent and unambiguous basis for scientific measurements and calculations, unlike relative scales like Celsius and Fahrenheit.
Water's Freezing Point in Kelvin: The Crucial 273.15 K
The freezing point of water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is precisely 273.15 Kelvin (K). This value is not arbitrarily chosen; it's directly derived from the relationship between the Kelvin and Celsius scales. Since 0°C is the freezing point of water on the Celsius scale, and the Kelvin scale is offset by 273.15, the freezing point in Kelvin is simply 0°C + 273.15 = 273.15 K.
This seemingly small difference in numerical value holds immense scientific significance. It allows for consistent and precise calculations involving thermodynamic processes, particularly those involving changes in state (like freezing or melting). The use of Kelvin ensures that calculations are not dependent on an arbitrary reference point.
Why Kelvin Matters in Scientific Calculations
Using the Kelvin scale in scientific calculations offers several advantages:
-
Avoiding Negative Values: Unlike Celsius, the Kelvin scale avoids negative values, simplifying calculations, especially those involving temperature differences or ratios.
-
Direct Proportionality: Many physical laws and equations, particularly in thermodynamics and gas laws, are expressed in terms of absolute temperature. Using Kelvin ensures a direct proportional relationship, simplifying calculations and interpretations.
-
Accuracy and Precision: The absolute nature of the Kelvin scale ensures greater accuracy and precision in scientific measurements and calculations. This is particularly important in fields such as cryogenics, material science, and astrophysics.
Applications of Water's Freezing Point in Kelvin
The knowledge of water's freezing point at 273.15 K has far-reaching applications across various disciplines:
1. Cryogenics and Low-Temperature Physics:
Cryogenics extensively uses the Kelvin scale to study materials and phenomena at extremely low temperatures. Precise temperature control near and below the freezing point of water is crucial in achieving and maintaining superconductivity, studying quantum phenomena, and developing advanced materials.
2. Meteorology and Climatology:
Meteorologists and climatologists use the Kelvin scale to model atmospheric processes, including cloud formation, precipitation, and temperature gradients. Understanding the freezing point of water in Kelvin is fundamental to predicting weather patterns and understanding climate change.
3. Chemistry and Biochemistry:
Chemical reactions and biochemical processes are highly sensitive to temperature. Expressing temperatures in Kelvin ensures consistency and accuracy in studying reaction kinetics, equilibrium constants, and other thermochemical properties. The freezing point of water serves as a crucial reference point in many experimental procedures and analyses.
4. Food Science and Engineering:
Freezing is a vital process in food preservation. Understanding the precise freezing point of water in Kelvin allows food scientists to optimize freezing conditions, minimizing ice crystal formation, and preserving food quality.
5. Environmental Science:
The freezing point of water is a crucial parameter in understanding ecological processes. Freezing and thawing cycles impact soil properties, water availability, and the survival of organisms. Precise measurements using the Kelvin scale improve the accuracy of environmental models and predictions.
6. Material Science and Engineering:
Many materials undergo phase transitions, like freezing or melting, near the freezing point of water. Understanding these transitions in Kelvin provides critical information for designing materials with desired properties. This knowledge is crucial in diverse areas from construction to aerospace engineering.
7. Medical Applications:
In medical applications, precise temperature control is crucial. Understanding the freezing point of water is essential in cryosurgery, cryopreservation of biological samples, and various medical imaging techniques.
Factors Affecting the Freezing Point of Water
While 273.15 K is the standard freezing point of water, several factors can slightly alter it:
-
Pressure: Increasing pressure slightly lowers the freezing point of water. This unusual behavior is due to the denser structure of liquid water compared to ice.
-
Impurities: Dissolved substances in water (salts, sugars, etc.) lower its freezing point. This phenomenon, known as freezing point depression, is utilized in various applications, such as de-icing roads and preserving food.
-
Altitude: At higher altitudes, where atmospheric pressure is lower, the freezing point of water is slightly elevated.
Conclusion: The Universal Importance of 273.15 K
The freezing point of water at 273.15 K is more than just a numerical value; it's a fundamental constant with widespread implications across diverse scientific and technological fields. The use of the Kelvin scale ensures precision, consistency, and facilitates accurate calculations and interpretations in various thermodynamic and physical phenomena. Understanding this seemingly simple concept unlocks a deeper appreciation for the intricate relationship between temperature, matter, and the processes that shape our world. From cryogenic research to food preservation and weather prediction, the knowledge of water's freezing point in Kelvin is an indispensable tool in numerous scientific and technological endeavors. The continued exploration and refinement of our understanding of this value will undoubtedly lead to further advancements in these fields and beyond.
Latest Posts
Latest Posts
-
The Process Of Dna Replication Occurs Just Before
Mar 21, 2025
-
How Many Hours Is 180 Min
Mar 21, 2025
-
What Is The Natural Log Of Infinity
Mar 21, 2025
-
Templates For Letter To Claim Deceased Accounts
Mar 21, 2025
-
What Is The Third Root Of 27
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point For Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
