Examples Of Polar And Non Polar Solvents
Juapaving
Mar 26, 2025 · 6 min read
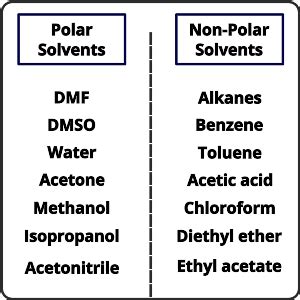
Table of Contents
Examples of Polar and Nonpolar Solvents: A Deep Dive
Understanding the polarity of solvents is crucial in chemistry, particularly in organic chemistry and biochemistry. The choice of solvent significantly impacts reaction rates, solubility, and the overall success of a chemical process. This comprehensive guide explores the concept of polarity in solvents, providing numerous examples of both polar and nonpolar solvents, and delving into the factors that determine their polarity. We’ll also examine practical applications and considerations for choosing the appropriate solvent for a given task.
What is Polarity in Solvents?
Polarity refers to the distribution of electrical charge within a molecule. In a polar molecule, the electrons are not shared equally between atoms, resulting in a partial positive charge (δ+) on one end and a partial negative charge (δ-) on the other. This uneven charge distribution creates a dipole moment. In contrast, a nonpolar molecule has a relatively even distribution of electrons, resulting in little to no dipole moment. The polarity of a solvent directly influences its ability to dissolve different types of solutes, following the principle "like dissolves like." Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
Factors Determining Solvent Polarity
Several factors contribute to a solvent's polarity:
1. Molecular Structure and Electronegativity:
The difference in electronegativity between atoms within a molecule is a primary determinant of polarity. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. A large electronegativity difference between atoms leads to a greater charge separation and thus higher polarity. For example, in water (H₂O), oxygen is significantly more electronegative than hydrogen, resulting in a polar molecule.
2. Molecular Shape and Symmetry:
Even if a molecule contains polar bonds, its overall polarity can be reduced or even eliminated by its molecular geometry. If the polar bonds are symmetrically arranged, their dipole moments can cancel each other out, resulting in a nonpolar molecule. For example, carbon dioxide (CO₂) has two polar C=O bonds, but its linear geometry causes the dipole moments to cancel, making it a nonpolar molecule.
3. Hydrogen Bonding:
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine). Hydrogen bonds are relatively strong intermolecular forces that significantly increase the polarity and boiling point of a solvent. Water is a prime example of a solvent with strong hydrogen bonding capabilities.
Examples of Polar Solvents
Polar solvents are characterized by their ability to dissolve polar solutes, such as salts, sugars, and many organic molecules containing polar functional groups (like alcohols, ketones, and carboxylic acids). Here are some common examples:
1. Water (H₂O):
Water is the most common and arguably the most important polar solvent. Its high polarity, due to the strong electronegativity of oxygen and its bent molecular geometry, allows it to dissolve a wide range of polar substances. Its ability to form hydrogen bonds further enhances its solvent properties.
2. Methanol (CH₃OH):
Methanol is a simple alcohol with a polar hydroxyl (-OH) group. This group contributes significantly to its polarity, making it a good solvent for many polar compounds. It's also miscible with water, meaning it can mix with water in all proportions.
3. Ethanol (CH₃CH₂OH):
Similar to methanol, ethanol is a polar solvent due to the presence of the hydroxyl group. It's frequently used as a solvent in various applications, including pharmaceuticals and cosmetics. It's also miscible with water.
4. Acetone (CH₃COCH₃):
Acetone is a ketone with a polar carbonyl group (C=O). Its polar nature makes it an excellent solvent for many organic compounds, including fats, oils, and resins.
5. Dimethyl Sulfoxide (DMSO):
DMSO is a highly polar aprotic solvent (meaning it doesn't donate protons). Its high polarity and ability to dissolve both polar and nonpolar compounds make it a versatile solvent in various chemical applications.
6. Acetonitrile (CH₃CN):
Acetonitrile is a polar aprotic solvent with a high dielectric constant. This makes it a suitable solvent for many ionic reactions.
7. Dimethylformamide (DMF):
DMF is another polar aprotic solvent frequently used in organic synthesis due to its high polarity and ability to dissolve a wide range of compounds.
Examples of Nonpolar Solvents
Nonpolar solvents are characterized by their inability to dissolve polar compounds. They primarily dissolve nonpolar solutes, such as fats, oils, and many hydrocarbons. Here are some common examples:
1. Hexane (C₆H₁₄):
Hexane is a straight-chain alkane with six carbon atoms. Its nonpolar nature makes it a good solvent for fats, oils, and other nonpolar organic compounds.
2. Heptane (C₇H₁₆):
Similar to hexane, heptane is a nonpolar alkane often used as a solvent in various applications, including extraction and chromatography.
3. Octane (C₈H₁₈):
Octane is another nonpolar alkane commonly found in gasoline. Its nonpolar nature limits its ability to dissolve polar compounds.
4. Benzene (C₆H₆):
Benzene is an aromatic hydrocarbon with a ring structure. Although it has polar C-H bonds, the symmetry of the molecule cancels out the dipole moments, rendering it essentially nonpolar. Note: Benzene is a known carcinogen and should be handled with extreme caution.
5. Toluene (C₇H₈):
Toluene is a methyl-substituted benzene. Like benzene, it's a relatively nonpolar solvent often used in organic chemistry.
6. Chloroform (CHCl₃):
While containing polar C-Cl bonds, chloroform's overall polarity is relatively low due to its symmetrical tetrahedral shape. It's considered a moderately polar solvent and often used as a solvent in various applications. It's also a potent anesthetic and should be handled carefully.
7. Carbon Tetrachloride (CCl₄):
Carbon tetrachloride is a nonpolar solvent with a tetrahedral structure. Although it contains polar C-Cl bonds, their symmetrical arrangement cancels out the dipole moments, making it a nonpolar solvent. It's considered toxic and is no longer widely used.
Practical Applications and Considerations
The choice of solvent is crucial for various chemical processes and applications. Here are some considerations:
-
Solubility: The most important factor is the solvent's ability to dissolve the solute of interest. Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
-
Reaction Conditions: The solvent can influence the rate and outcome of chemical reactions. For example, polar solvents can stabilize ionic intermediates, while nonpolar solvents may favor certain reaction pathways.
-
Safety: Some solvents are toxic, flammable, or harmful to the environment. Choosing safe solvents is crucial for both personal safety and environmental protection.
-
Cost: Solvents vary considerably in cost. Choosing an economically viable solvent is often a practical consideration.
-
Ease of Purification: The solvent's ease of removal from the reaction mixture after the reaction is complete can influence the selection process.
-
Boiling Point: The boiling point of the solvent impacts the ease of solvent removal during the workup process.
Conclusion
Understanding the polarity of solvents is fundamental to successful chemical work. The "like dissolves like" principle guides the selection of appropriate solvents for different solutes. This article has provided numerous examples of both polar and nonpolar solvents, highlighting the factors influencing their polarity and considering their practical applications. Choosing the correct solvent requires careful consideration of various aspects, including solubility, reaction conditions, safety, cost, and ease of purification. Always refer to safety data sheets (SDS) before handling any chemical, especially solvents. Remember that safe practices are paramount in any laboratory setting.
Latest Posts
Latest Posts
-
Five Letter Word Starts With Vi
Mar 29, 2025
-
Why Is Air A Homogeneous Mixture
Mar 29, 2025
-
How To Simplify Square Root Of 80
Mar 29, 2025
-
How Do You Separate Oil And Vinegar
Mar 29, 2025
-
Is 2 Cm The Same As 1 Inch
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Examples Of Polar And Non Polar Solvents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
