Electron Pair Geometry Vs Molecular Geometry
Juapaving
Mar 25, 2025 · 6 min read
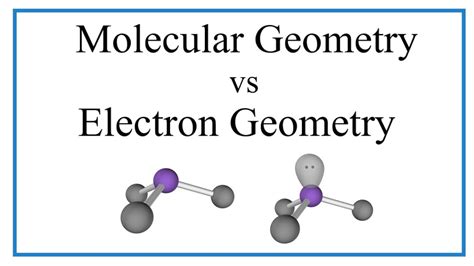
Table of Contents
Electron Pair Geometry vs. Molecular Geometry: A Comprehensive Guide
Understanding the three-dimensional arrangement of atoms within a molecule is crucial in chemistry. This arrangement dictates a molecule's properties, including its reactivity, polarity, and physical state. Two key concepts, electron pair geometry and molecular geometry, are essential for visualizing and predicting these arrangements. While often confused, they are distinct yet interconnected concepts that provide a complete picture of a molecule's structure. This comprehensive guide will delve into the differences and similarities between electron pair geometry and molecular geometry, providing you with the tools to confidently predict the shape of any molecule.
What is Electron Pair Geometry?
Electron pair geometry describes the arrangement of all electron pairs surrounding the central atom in a molecule, including both bonding pairs (electrons shared between atoms) and lone pairs (electrons not involved in bonding). It considers the repulsive forces between all electron pairs, regardless of whether they are bonding or non-bonding. The repulsion between electron pairs dictates their spatial arrangement to minimize these repulsions and achieve maximum stability. The fundamental principle guiding this arrangement is VSEPR theory (Valence Shell Electron Pair Repulsion theory).
VSEPR Theory: The Foundation of Electron Pair Geometry
VSEPR theory postulates that electron pairs around a central atom will arrange themselves to be as far apart as possible to minimize electron-electron repulsion. This results in specific geometric shapes depending on the number of electron pairs. Here are some key electron pair geometries predicted by VSEPR theory:
-
Linear (AX₂): Two electron pairs arrange themselves 180° apart. Examples include BeCl₂ and CO₂.
-
Trigonal Planar (AX₃): Three electron pairs arrange themselves 120° apart in a flat, triangular plane. Examples include BF₃ and SO₃.
-
Tetrahedral (AX₄): Four electron pairs arrange themselves 109.5° apart in a three-dimensional tetrahedral shape. Examples include CH₄ and SiCl₄.
-
Trigonal Bipyramidal (AX₅): Five electron pairs arrange themselves in a trigonal bipyramidal geometry. Three electron pairs are in the equatorial plane (120° apart), and two are axial (180° apart). Examples include PCl₅ and SF₄.
-
Octahedral (AX₆): Six electron pairs arrange themselves 90° apart in an octahedral shape. Examples include SF₆ and XeF₆.
What is Molecular Geometry?
Molecular geometry, on the other hand, only considers the arrangement of atoms in a molecule. It focuses solely on the positions of the atoms bonded to the central atom and ignores the lone pairs of electrons. While electron pair geometry accounts for all electron pairs, molecular geometry focuses only on the atoms and their spatial relationships. The presence of lone pairs significantly influences molecular geometry because they occupy space and exert repulsive forces on the bonding pairs, distorting the ideal geometries predicted by electron pair geometry.
Lone Pairs and their Impact on Molecular Geometry
Lone pairs are more diffuse than bonding pairs, meaning their electron density is spread over a larger volume. This results in stronger repulsive forces compared to bonding pairs. Consequently, lone pairs push the bonding pairs closer together, altering the ideal bond angles and resulting in different molecular shapes.
Let's illustrate this with some examples:
-
Consider CH₄ (methane): Both electron pair geometry and molecular geometry are tetrahedral. There are four bonding pairs and no lone pairs; thus, the arrangement of atoms mirrors the arrangement of all electron pairs.
-
Consider NH₃ (ammonia): The electron pair geometry is tetrahedral (four electron pairs around the nitrogen atom). However, one of these pairs is a lone pair. This lone pair repels the three bonding pairs, compressing the bond angles slightly from the ideal 109.5° to approximately 107°. Therefore, the molecular geometry is trigonal pyramidal.
-
Consider H₂O (water): The electron pair geometry is tetrahedral (four electron pairs around the oxygen atom). However, two of these pairs are lone pairs. These lone pairs exert stronger repulsive forces, compressing the H-O-H bond angle from the ideal 109.5° to approximately 104.5°. The molecular geometry is bent or V-shaped.
Key Differences Between Electron Pair Geometry and Molecular Geometry
| Feature | Electron Pair Geometry | Molecular Geometry |
|---|---|---|
| Focus | Arrangement of all electron pairs (bonding & lone pairs) | Arrangement of atoms only |
| Lone Pairs | Included in the determination of geometry | Ignored in the determination of geometry |
| Shape | Determined by the total number of electron pairs | Determined by the number of bonding pairs and lone pairs |
| Ideal Angles | Follows VSEPR theory perfectly (e.g., 109.5° for tetrahedral) | Deviates from ideal angles due to lone pair repulsion |
Predicting Molecular Geometry: A Step-by-Step Approach
To accurately predict the molecular geometry of a molecule, follow these steps:
-
Draw the Lewis Structure: Determine the number of valence electrons for each atom and arrange them to satisfy the octet rule (or duet rule for hydrogen).
-
Determine the Electron Pair Geometry: Count the total number of electron pairs around the central atom (both bonding and lone pairs). Use VSEPR theory to predict the electron pair geometry based on the number of electron pairs.
-
Determine the Molecular Geometry: Count the number of bonding pairs and lone pairs. The presence of lone pairs will distort the ideal geometry predicted by the electron pair geometry. Consider the stronger repulsive forces of lone pairs. Use appropriate terminology to describe the molecular geometry (e.g., linear, bent, trigonal pyramidal, tetrahedral, trigonal bipyramidal, octahedral, square pyramidal, square planar).
Examples: Putting it all Together
Let's illustrate this with a few more examples:
1. PCl₅ (Phosphorus Pentachloride):
-
Lewis Structure: Phosphorus has 5 valence electrons, and each chlorine has 7. The Lewis structure shows phosphorus bonded to five chlorine atoms, with no lone pairs on the phosphorus atom.
-
Electron Pair Geometry: Five electron pairs around the central phosphorus atom result in a trigonal bipyramidal electron pair geometry.
-
Molecular Geometry: Since there are five bonding pairs and zero lone pairs, the molecular geometry is also trigonal bipyramidal.
2. SF₄ (Sulfur Tetrafluoride):
-
Lewis Structure: Sulfur has 6 valence electrons, and each fluorine has 7. The Lewis structure shows sulfur bonded to four fluorine atoms, with one lone pair on the sulfur atom.
-
Electron Pair Geometry: Five electron pairs (four bonding pairs and one lone pair) around the central sulfur atom result in a trigonal bipyramidal electron pair geometry.
-
Molecular Geometry: The presence of the lone pair distorts the geometry. The lone pair occupies an equatorial position (to minimize repulsion), resulting in a see-saw molecular geometry.
3. XeF₂ (Xenon Difluoride):
-
Lewis Structure: Xenon has 8 valence electrons, and each fluorine has 7. The Lewis structure shows xenon bonded to two fluorine atoms with three lone pairs on the xenon atom.
-
Electron Pair Geometry: Five electron pairs (two bonding pairs and three lone pairs) around the central xenon atom result in a trigonal bipyramidal electron pair geometry.
-
Molecular Geometry: The three lone pairs occupy the equatorial positions, resulting in a linear molecular geometry.
Conclusion: A Unified Perspective
Electron pair geometry and molecular geometry are fundamental concepts for understanding the three-dimensional structure of molecules. While distinct, they are closely related, with electron pair geometry providing the foundation for predicting molecular geometry. By understanding VSEPR theory and the influence of lone pairs, you can accurately predict the shape of a molecule and gain valuable insights into its properties. This knowledge is crucial for various fields, including organic chemistry, inorganic chemistry, biochemistry, and materials science. Mastering these concepts will significantly enhance your understanding of molecular structure and reactivity.
Latest Posts
Latest Posts
-
What Is 25 Cm In Inches
Mar 26, 2025
-
Which Of The Following Statements Describes The Process Of Globalization
Mar 26, 2025
-
What Are The Greatest Common Factors Of 48
Mar 26, 2025
-
The Unit Of Energy In S I Units Is
Mar 26, 2025
-
How Does Temperature Relate To Kinetic Energy
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Electron Pair Geometry Vs Molecular Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
