Drawbacks Of Rutherford Model Of Atom
Juapaving
Apr 06, 2025 · 6 min read
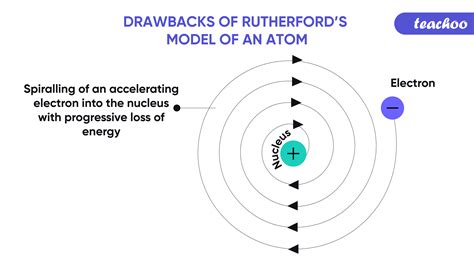
Table of Contents
Drawbacks of the Rutherford Model of Atom: Why it Couldn't Explain Atomic Structure
The Rutherford model of the atom, proposed by Ernest Rutherford in 1911, revolutionized our understanding of atomic structure. His gold foil experiment demonstrated that the atom wasn't a solid, indivisible sphere as previously believed, but rather contained a small, dense, positively charged nucleus surrounded by mostly empty space where electrons resided. While groundbreaking, the Rutherford model had significant limitations and couldn't fully explain the observed properties of atoms. This article delves into the major drawbacks of the Rutherford model, highlighting its inconsistencies with experimental evidence and paving the way for the development of more sophisticated atomic models.
Instability of the Atom: The Electron's Predicament
One of the most significant drawbacks of the Rutherford model was its inability to explain the stability of the atom. According to classical electromagnetism, a charged particle undergoing acceleration, like an electron orbiting the nucleus, should continuously radiate electromagnetic energy. This continuous energy loss would cause the electron to spiral into the nucleus, leading to the collapse of the atom within a fraction of a second. This clearly contradicted the observed stability of matter. Atoms don't spontaneously collapse; they exist for extended periods. The Rutherford model failed to offer an explanation for this crucial aspect of atomic behavior.
The Classical Physics Failure
The problem stemmed from the application of classical physics principles to the subatomic realm. Classical physics, successful in describing macroscopic phenomena, proved inadequate in explaining the behavior of particles at the atomic scale. The Rutherford model relied on classical mechanics and electromagnetism, neglecting the emerging quantum mechanics that would ultimately provide the correct description.
Lack of Explanation for Atomic Spectra
Another major drawback was the Rutherford model's failure to explain the discrete nature of atomic spectra. When atoms are heated or subjected to electrical discharge, they emit light of specific wavelengths. This light, when passed through a prism, produces a distinct line spectrum—a series of bright lines at specific frequencies, unique to each element. The continuous emission of energy predicted by the Rutherford model should have resulted in a continuous spectrum, not the discrete lines observed. The model couldn't account for this fundamental characteristic of atomic behavior, leaving a critical gap in our understanding of atomic structure and its interaction with light.
The Mystery of Discrete Lines
The sharp, distinct lines in atomic spectra implied that atoms could only exist in certain specific energy states. The electrons couldn't orbit the nucleus at any arbitrary distance or energy level. This quantization of energy, a cornerstone of quantum mechanics, was completely absent from the Rutherford model. The model couldn't explain why only specific wavelengths were emitted, nor could it predict the specific wavelengths for a given element.
Inability to Explain Chemical Bonding
The Rutherford model also failed to explain the nature of chemical bonding. Atoms combine to form molecules through chemical bonds, a phenomenon crucial to chemistry and materials science. The model offered no mechanism to explain how atoms interact and bond together. While it could suggest electrostatic attraction between the positive nucleus and negative electrons, it lacked the framework to describe the specific arrangements and interactions leading to stable molecular structures.
The Unanswered Question of Molecular Structure
The formation of molecules with specific geometries and properties couldn't be explained by simply considering the electrostatic forces between the nucleus and electrons of different atoms. The model provided no insight into the sharing or transfer of electrons that underpins chemical bonding, a critical aspect of chemical reactions and molecular stability. A more refined model was needed to unravel the mysteries of chemical bonding and molecular structure.
Limitations in Describing Isotopes
The Rutherford model, while acknowledging the existence of a nucleus with positive charge, didn't adequately address the concept of isotopes. Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This difference in neutron number affects the atomic mass, yet the Rutherford model offered no explanation for the existence of isotopes or how they differed structurally from each other.
Beyond Protons and Electrons
The model focused primarily on protons and electrons, neglecting the role of neutrons in determining atomic properties. The discovery of neutrons later significantly impacted our understanding of the nucleus and its composition, highlighting the inadequacy of the Rutherford model in encompassing all the subatomic particles and their influence on atomic behavior.
No Explanation for the Zeeman Effect and Stark Effect
The Rutherford model couldn't explain the Zeeman effect and Stark effect. The Zeeman effect refers to the splitting of spectral lines when an atom is placed in a strong magnetic field, while the Stark effect involves the splitting of spectral lines in a strong electric field. These effects demonstrate the interaction of atoms with external fields, influencing the energy levels of electrons. The Rutherford model lacked the framework to explain these phenomena because it didn't incorporate the quantization of angular momentum and the magnetic moment associated with electron orbits.
External Field Influences on Atomic Spectra
The splitting of spectral lines in the presence of external fields indicated a more complex interaction between the atom's internal structure and external forces than the Rutherford model could account for. These effects further highlighted the limitations of applying classical physics to the atomic world.
The Path to Quantum Mechanics
The shortcomings of the Rutherford model served as a catalyst for the development of quantum mechanics. Physicists recognized the need for a new theoretical framework that could address the observed inconsistencies and incorporate concepts like quantization of energy, angular momentum, and wave-particle duality. The Bohr model, proposed in 1913, represented a significant improvement, introducing quantized energy levels for electrons, but it still had its own limitations. Later, the Schrödinger model and quantum mechanics provided a much more complete and accurate description of atomic structure and behavior.
Beyond Classical Limitations
The limitations of the Rutherford model weren't merely minor inconsistencies. They revealed the fundamental limitations of classical physics in the realm of the atom and emphasized the need for a revolutionary new theory – a theory that would eventually lead to quantum mechanics, which successfully describes the quantum world, including atomic structure, bonding, and interactions with external fields. The legacy of the Rutherford model, therefore, lies not only in its contributions but also in highlighting the limitations of classical physics, thereby paving the way for a more profound understanding of the atomic world.
Conclusion: A Stepping Stone to a Deeper Understanding
The Rutherford model, despite its shortcomings, was a crucial step forward in our understanding of the atom. It established the existence of the nucleus, a central concept in modern atomic theory. However, its inability to explain atomic stability, discrete spectra, chemical bonding, isotopes, and the Zeeman and Stark effects ultimately demonstrated its limitations. These limitations highlighted the need for a paradigm shift in our understanding of matter at the atomic level, leading to the development of quantum mechanics and a more accurate description of atomic structure and behavior. The model serves as a testament to the iterative nature of scientific progress—building upon past discoveries to refine our understanding of the universe. The flaws of the Rutherford model ultimately propelled the field forward, revealing the complexities of the quantum world and paving the way for our current comprehensive understanding of atomic structure.
Latest Posts
Latest Posts
-
3 Meters Is How Many Centimeters
Apr 07, 2025
-
Equilateral Triangle Inscribed In A Circle
Apr 07, 2025
-
What Is Larger 2 8 Or 3 4
Apr 07, 2025
-
16 Meters Is How Many Feet
Apr 07, 2025
-
Words With A Z In It
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Drawbacks Of Rutherford Model Of Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
