Does Carbon Follow The Octet Rule
Juapaving
Mar 18, 2025 · 6 min read
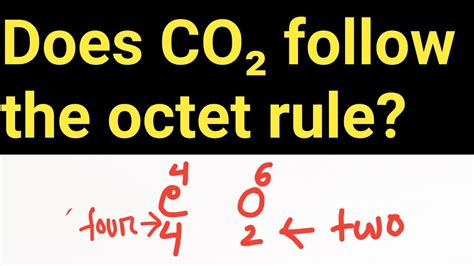
Table of Contents
Does Carbon Follow the Octet Rule? A Deep Dive into Carbon's Bonding Behavior
Carbon, the backbone of life and a cornerstone of organic chemistry, presents a fascinating case study when examining the octet rule. While often presented as a steadfast follower of this fundamental principle of chemical bonding, the reality is more nuanced and intriguing. This article will explore the extent to which carbon adheres to the octet rule, examining exceptions and exploring the implications for its diverse chemical behavior.
Understanding the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their outermost shell (valence shell). This configuration provides stability, mirroring the electron arrangement of noble gases, which are famously unreactive. This stability arises from the complete filling of the s and p orbitals in the valence shell.
However, it's crucial to remember that the octet rule is a guideline, not an absolute law. Many elements, including carbon itself, exhibit exceptions under certain circumstances.
Carbon's Electron Configuration and Bonding
Carbon possesses six electrons, with its electronic configuration being 1s²2s²2p². This means it has four electrons in its outermost shell (the second shell). To achieve a stable octet, carbon needs to gain four electrons or lose four electrons. Losing four electrons would require an enormous amount of energy, making it highly improbable. Therefore, carbon predominantly achieves stability by sharing its four valence electrons through covalent bonding.
This covalent bonding capability is the foundation of carbon's incredible versatility and ability to form a vast array of molecules. Through sharing electrons, carbon can form single, double, and triple bonds with itself and other elements, leading to the rich diversity of organic compounds.
Carbon Compounds Obeying the Octet Rule
In a significant number of its compounds, carbon flawlessly adheres to the octet rule. Consider methane (CH₄):
- Methane (CH₄): Carbon shares one electron with each of the four hydrogen atoms, forming four single covalent bonds. This results in carbon having eight electrons in its valence shell – a complete octet.
Similarly, ethane (C₂H₆), propane (C₃H₈), and countless other alkanes follow the octet rule. Each carbon atom shares its four valence electrons with other carbon atoms and hydrogen atoms, completing its octet in every instance.
Other examples include:
-
Carbon dioxide (CO₂): Carbon forms two double bonds with two oxygen atoms, leading to a complete octet around the carbon atom. Each oxygen atom also achieves a complete octet.
-
Ethanol (C₂H₅OH): Each carbon atom in ethanol forms the necessary bonds to complete its octet.
These examples demonstrate the prevalent adherence of carbon to the octet rule in a wide range of stable, common organic compounds. This consistent adherence underscores the rule's importance in predicting carbon's bonding behavior.
Exceptions to the Octet Rule for Carbon: Electron-Deficient Compounds
While carbon often follows the octet rule, there are notable exceptions, primarily found in electron-deficient compounds. These are molecules where carbon has fewer than eight electrons in its valence shell. The most significant examples include carbocations and carbenes.
Carbocations
Carbocations are positively charged carbon species with only six electrons in their valence shell. They are highly reactive intermediates in many organic reactions. The positive charge arises from the loss of an electron, leaving a carbon atom with three bonds and an empty p-orbital. The instability of carbocations makes them highly electrophilic, readily seeking out electron-rich species to complete their octet.
Examples include:
-
Methyl carbocation (CH₃⁺): Carbon has only three bonds, resulting in a sextet instead of an octet.
-
Tertiary butyl carbocation ((CH₃)₃C⁺): The positive charge is stabilized by the electron-donating methyl groups but still represents a significant departure from the octet rule.
Carbenes
Carbenes are neutral molecules with a divalent carbon atom possessing only six valence electrons. This is because the carbon atom is bonded to only two other atoms, leaving two unshared electrons. These unshared electrons can be paired (singlet carbene) or unpaired (triplet carbene), influencing their reactivity. Carbenes are highly reactive species due to their electron deficiency.
Examples include:
-
Dichlorocarbene (:CCl₂): A reactive intermediate formed in various reactions.
-
Methylene (:CH₂): The simplest carbene, a highly reactive species.
These electron-deficient species showcase carbon's ability to exist in states where it violates the octet rule, highlighting the rule's limitations as an absolute predictor of bonding behavior. Their high reactivity is a direct consequence of their incomplete octets.
Exceptions to the Octet Rule for Carbon: Hypervalent Compounds
While less common than electron-deficient exceptions, carbon can also participate in what are termed hypervalent compounds. These involve having more than eight electrons in the valence shell. While rare for carbon, some organometallic compounds show characteristics of hypervalency, involving expanded valence shells. These situations usually involve the participation of d-orbitals, which are not typically involved in carbon's bonding in typical organic compounds. The involvement of d-orbitals allows for an expansion of the octet. However, debate still exists about the true nature of bonding in these specific cases.
Factors Influencing Carbon's Adherence to the Octet Rule
Several factors influence whether carbon will strictly follow the octet rule:
-
Electronegativity of bonded atoms: Bonding with highly electronegative atoms can sometimes induce a slight deviation from the octet rule due to electron polarization.
-
Resonance structures: In molecules with resonance structures, the actual electronic distribution is a hybrid of contributing structures, potentially leading to slight deviations from the strict octet rule for specific carbon atoms.
-
Steric hindrance: Bulky groups surrounding a carbon atom can influence its bonding behavior, sometimes affecting its ability to attain a complete octet.
Conclusion: The Octet Rule as a Useful Guideline for Carbon
In summary, while carbon frequently follows the octet rule, it's essential to recognize the notable exceptions. Electron-deficient species such as carbocations and carbenes provide clear examples of situations where carbon deviates from this guideline. Hypervalent compounds, although less common, also present a departure from the octet rule. Understanding these exceptions provides a more complete understanding of carbon's rich and versatile chemistry.
The octet rule serves as a valuable tool for predicting the bonding behavior of carbon in many situations, allowing for the prediction of molecular shapes and reactivity. However, it should not be considered an inviolable law. The exceptions illustrate the flexibility and adaptability of carbon's bonding capabilities, which ultimately underpin the incredible diversity of organic molecules found in nature and synthesized in laboratories. A thorough grasp of both the rule and its exceptions is critical for a comprehensive understanding of carbon chemistry. The ability to predict and explain these deviations contributes significantly to the advancement of organic chemistry and related fields.
Latest Posts
Latest Posts
-
What Is The Symbol Of Energy
Mar 18, 2025
-
How Many Minerals Are Considered To Be Essential
Mar 18, 2025
-
Natural Boundary Between France And Itsly
Mar 18, 2025
-
How Many Side Does A Octagon Have
Mar 18, 2025
-
What Kingdom Does Euglena Belong To
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Does Carbon Follow The Octet Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
