Do Nonmetals Form Anions Or Cations
Juapaving
Mar 10, 2025 · 5 min read
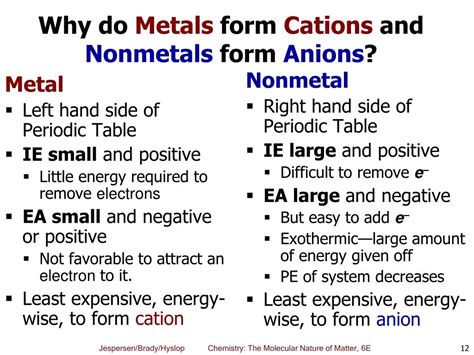
Table of Contents
Do Nonmetals Form Anions or Cations? Understanding Ionic Bonding and Electronegativity
The question of whether nonmetals form anions or cations is fundamental to understanding chemical bonding and the behavior of elements. The short answer is that nonmetals predominantly form anions, meaning negatively charged ions. This behavior stems from their electronic configurations and their tendency to gain electrons to achieve a stable electron configuration, typically resembling that of a noble gas. Let's delve deeper into the reasons behind this, exploring the concepts of electronegativity, ionic bonding, and the exceptions that exist.
Understanding Electronegativity: The Driving Force Behind Anion Formation
Electronegativity is a crucial concept in predicting the behavior of elements in chemical reactions. It represents an atom's ability to attract electrons towards itself within a chemical bond. Nonmetals generally exhibit high electronegativity. This means they possess a strong attraction for electrons, making them more likely to gain electrons rather than lose them. Conversely, metals have low electronegativity and tend to lose electrons easily.
This difference in electronegativity is the driving force behind the formation of ionic bonds. When a nonmetal interacts with a metal, the high electronegativity of the nonmetal overcomes the low electronegativity of the metal. The nonmetal effectively "pulls" electrons away from the metal atom, resulting in the formation of a positively charged metal cation and a negatively charged nonmetal anion.
Visualizing the Electron Transfer: A Simple Example
Consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na), an alkali metal, has one valence electron. Chlorine (Cl), a halogen, has seven valence electrons. Chlorine, with its higher electronegativity, readily accepts the single valence electron from sodium. This transfer results in:
- Na: Loses one electron, becoming a positively charged sodium cation (Na⁺).
- Cl: Gains one electron, becoming a negatively charged chloride anion (Cl⁻).
The electrostatic attraction between the oppositely charged ions forms the ionic bond that holds the sodium chloride crystal lattice together.
The Octet Rule and Stable Electron Configurations
The tendency of nonmetals to form anions is closely tied to the octet rule. This rule states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell containing eight electrons, similar to the stable electron configuration of noble gases. Since nonmetals typically have 5, 6, or 7 valence electrons, it's energetically more favorable for them to gain electrons (1, 2, or 3 respectively) to complete their octet, rather than lose a larger number of electrons.
Exceptions to the Octet Rule: Expanding the Possibilities
While the octet rule is a useful guideline, it's not without exceptions. Some nonmetals, particularly those in the third period and beyond, can accommodate more than eight electrons in their valence shell, forming what are known as expanded octets. This is because they possess empty d-orbitals that can participate in bonding. Examples include phosphorus (P) and sulfur (S), which can form anions with more than eight electrons.
Specific Examples of Nonmetal Anion Formation
Let's examine some specific examples to illustrate the common anions formed by nonmetals:
-
Group 17 (Halogens): These elements (fluorine, chlorine, bromine, iodine, astatine) readily gain one electron to form -1 anions (F⁻, Cl⁻, Br⁻, I⁻, At⁻). Their high electronegativity makes them exceptionally good at attracting electrons.
-
Group 16 (Chalcogens): These elements (oxygen, sulfur, selenium, tellurium, polonium) typically gain two electrons to form -2 anions (O²⁻, S²⁻, Se²⁻, Te²⁻, Po²⁻). Oxygen, in particular, is a highly electronegative element and plays a crucial role in numerous chemical compounds.
-
Group 15 (Pnictogens): Elements in this group (nitrogen, phosphorus, arsenic, antimony, bismuth) can gain three electrons to form -3 anions (N³⁻, P³⁻, As³⁻, Sb³⁻, Bi³⁻). However, simple -3 anions of nitrogen and phosphorus are less common than their involvement in covalent compounds.
-
Group 14 (Carbon Group): Elements in this group (carbon, silicon, germanium, tin, lead) generally do not form simple anions. Carbon is particularly well known for forming covalent bonds. While silicon and other members can form anions under certain conditions, they are less prevalent than those of groups 15-17.
When Nonmetals Might Seem to Form Cations: Covalent Bonding and Molecular Compounds
It's important to note that while nonmetals predominantly form anions in ionic compounds, they primarily form covalent bonds with each other. In covalent bonds, atoms share electrons rather than transferring them completely. Therefore, the concept of "cation" and "anion" isn't strictly applicable in the same way to covalent compounds. While we might assign oxidation states to atoms in covalent molecules, these states do not necessarily reflect the existence of distinct cations and anions.
For instance, consider carbon dioxide (CO₂). Carbon and oxygen share electrons to form double bonds. While we can assign oxidation states (+4 for carbon and -2 for each oxygen), this doesn't imply the presence of a C⁴⁺ cation and two O²⁻ anions. The molecule exists as a neutral entity with a covalent structure.
The Rare Cases: Nonmetals in Unusual Chemical Environments
Under extremely unusual conditions, it is theoretically possible for a nonmetal atom to lose electrons and behave as a cation. This is highly uncommon and usually requires very specific and extreme circumstances, such as extremely high temperatures or reactions involving highly reactive species. Such instances are far from the norm and shouldn't be considered typical nonmetal behavior.
Conclusion: Understanding the Predominant Trend
In summary, nonmetals overwhelmingly form anions due to their high electronegativity and their tendency to gain electrons to achieve stable electron configurations. This behavior is fundamental to the formation of ionic compounds and explains the widespread presence of nonmetal anions in various chemical contexts. While exceptions exist, such as expanded octets or unusual reaction conditions, the general trend remains firmly established. Understanding the principles of electronegativity, ionic bonding, and the octet rule provides a robust framework for predicting the behavior of nonmetals in chemical reactions.
Latest Posts
Latest Posts
-
Whats The Square Root Of 576
Mar 11, 2025
-
A Plasma Protein Essential For Blood Coagulation Is
Mar 11, 2025
-
What Is The Prime Factorization 140
Mar 11, 2025
-
In The Figure What Is The Value Of X
Mar 11, 2025
-
What Is Xxviii In Roman Numerals
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about Do Nonmetals Form Anions Or Cations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
