Do Bases Accept Or Donate Protons
Juapaving
Mar 23, 2025 · 6 min read
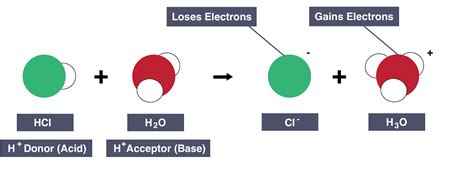
Table of Contents
Do Bases Accept or Donate Protons? Understanding Brønsted-Lowry Theory
The question of whether bases accept or donate protons is fundamental to understanding acid-base chemistry. The answer, simply put, is that bases accept protons. This definition stems from the Brønsted-Lowry theory, a cornerstone of modern acid-base chemistry. This article will delve deeply into this concept, exploring the Brønsted-Lowry definition, contrasting it with other acid-base theories, and examining various examples of bases and their proton-accepting behavior. We'll also explore the nuances of strong and weak bases and the relationship between conjugate acid-base pairs.
The Brønsted-Lowry Definition: The Foundation of Proton Acceptance
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, provides a comprehensive framework for understanding acid-base reactions. Unlike the simpler Arrhenius theory (which defines acids as substances that produce H⁺ ions in water and bases as substances that produce OH⁻ ions), the Brønsted-Lowry theory focuses on the transfer of protons (H⁺ ions).
According to this theory:
- An acid is a proton donor. It donates a proton (H⁺) to another species.
- A base is a proton acceptor. It accepts a proton (H⁺) from another species.
This definition is significantly broader than the Arrhenius definition because it doesn't require the presence of water. Acid-base reactions can occur in various solvents or even in the gas phase, as long as a proton transfer takes place.
Key takeaway: The core characteristic of a Brønsted-Lowry base is its ability to accept a proton. This acceptance often involves the formation of a new bond between the base and the proton.
Examples of Proton Acceptance by Bases
Let's illustrate the proton-accepting nature of bases with several examples:
1. Ammonia (NH₃) reacting with water (H₂O)
Ammonia, a common weak base, reacts with water according to the following equation:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
In this reaction, ammonia acts as a base because it accepts a proton from water. Water, in this case, acts as an acid, donating a proton to ammonia. The resulting products are the ammonium ion (NH₄⁺) and the hydroxide ion (OH⁻). The equilibrium arrow indicates that the reaction is reversible.
2. Hydroxide ion (OH⁻) reacting with a strong acid like HCl
The hydroxide ion is a strong base. Its reaction with hydrochloric acid (HCl) is a classic example of a Brønsted-Lowry acid-base reaction:
OH⁻(aq) + HCl(aq) → H₂O(l) + Cl⁻(aq)
Here, the hydroxide ion directly accepts a proton from the HCl molecule, forming water and the chloride ion. This reaction proceeds essentially to completion because HCl is a strong acid and OH⁻ is a strong base.
3. Carbonates (CO₃²⁻) reacting with acids
Carbonate ions are also strong bases. They can accept protons from acids like carbonic acid (H₂CO₃):
CO₃²⁻(aq) + H₂CO₃(aq) → 2HCO₃⁻(aq)
In this reaction, the carbonate ion accepts a proton from carbonic acid, forming bicarbonate ions (HCO₃⁻). This reaction is crucial in many natural processes, including buffering systems in the blood.
Strong vs. Weak Bases: The Extent of Proton Acceptance
The extent to which a base accepts a proton determines whether it's classified as strong or weak.
Strong Bases: Complete Proton Acceptance
Strong bases are those that completely dissociate in water, meaning they readily accept protons and essentially transfer them completely to water to form hydroxide ions. Examples include hydroxides of Group 1 and 2 metals like NaOH (sodium hydroxide), KOH (potassium hydroxide), and Ca(OH)₂ (calcium hydroxide). These bases have a high affinity for protons.
Weak Bases: Partial Proton Acceptance
Weak bases only partially dissociate in water. They accept protons to a lesser extent compared to strong bases, resulting in an equilibrium between the base and its conjugate acid. Examples include ammonia (NH₃), amines (organic compounds containing nitrogen), and many other organic compounds with lone pairs of electrons capable of accepting a proton.
The strength of a weak base is quantified by its base dissociation constant (Kb), which is the equilibrium constant for the reaction of the base with water. A higher Kb value indicates a stronger weak base.
Conjugate Acid-Base Pairs: A Key Concept
The Brønsted-Lowry theory introduces the concept of conjugate acid-base pairs. When an acid donates a proton, the species remaining is its conjugate base. Conversely, when a base accepts a proton, the resulting species is its conjugate acid.
For example, in the reaction between ammonia and water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
- NH₃ is the base, and NH₄⁺ is its conjugate acid.
- H₂O is the acid, and OH⁻ is its conjugate base.
Understanding conjugate acid-base pairs is crucial for predicting the outcome of acid-base reactions and understanding the behavior of buffer solutions.
Beyond Brønsted-Lowry: Other Acid-Base Theories
While the Brønsted-Lowry theory is widely used and extremely useful, it's not the only acid-base theory. The Lewis theory provides an even broader definition of acids and bases.
Lewis Theory: Electron Pair Acceptance
In the Lewis theory, an acid is defined as an electron-pair acceptor, and a base is defined as an electron-pair donor. This definition encompasses a wider range of reactions than the Brønsted-Lowry theory because it doesn't necessarily involve proton transfer. Many reactions involving the formation of coordinate covalent bonds can be considered Lewis acid-base reactions. For example, the reaction between boron trifluoride (BF₃, a Lewis acid) and ammonia (NH₃, a Lewis base) is a classic example of a Lewis acid-base reaction.
Practical Applications and Significance
The understanding of bases and their proton-accepting nature is crucial across various scientific disciplines and industries:
-
Medicine: Many drugs and biological molecules act as acids or bases, affecting pH levels in the body. Understanding acid-base reactions is critical in pharmacology and physiology.
-
Environmental Science: Acid rain, caused by the release of acidic pollutants into the atmosphere, affects soil and water chemistry. The use of bases can be crucial in remediating the effects of acid rain.
-
Industrial Chemistry: Acid-base reactions are fundamental in many industrial processes, including the production of fertilizers, detergents, and pharmaceuticals.
-
Analytical Chemistry: Acid-base titrations are widely used to determine the concentration of unknown solutions.
Conclusion: The Crucial Role of Proton Acceptance
In summary, bases are defined by their ability to accept protons according to the Brønsted-Lowry theory. This proton acceptance is a fundamental aspect of acid-base chemistry, influencing reaction equilibrium, strength of bases, and the formation of conjugate acid-base pairs. Understanding this concept is essential for comprehending a vast array of chemical processes and their applications in diverse fields. The distinction between strong and weak bases underscores the varying degrees of proton acceptance, significantly impacting the reaction's outcome and equilibrium position. While the Brønsted-Lowry theory provides a powerful framework, the Lewis theory offers a more comprehensive perspective on acid-base interactions, expanding the scope beyond simple proton transfer. The significant practical applications of understanding proton acceptance highlight the importance of this fundamental concept in science and technology.
Latest Posts
Latest Posts
-
Common Factors Of 20 And 40
Mar 25, 2025
-
How Many Lines Of Symmetry Does A Rectangle Has
Mar 25, 2025
-
Lowest Common Denominator Of 9 And 12
Mar 25, 2025
-
Is 15 A Prime Or Composite Number
Mar 25, 2025
-
Facilitated Diffusion And Active Transport Differ In That
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Do Bases Accept Or Donate Protons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
