Differentiate Between Exothermic And Endothermic Reactions
Juapaving
Mar 19, 2025 · 6 min read
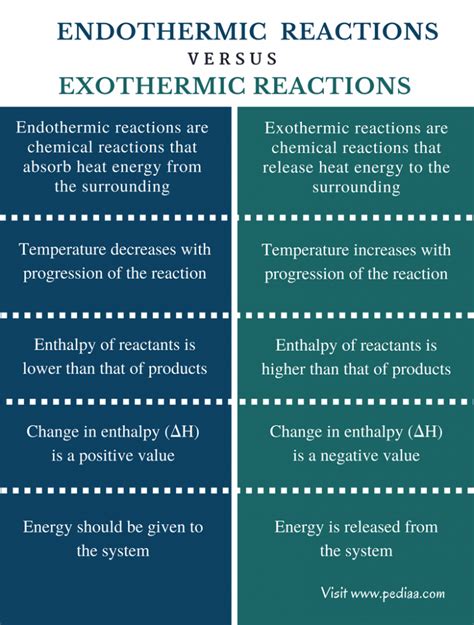
Table of Contents
Differentiating Between Exothermic and Endothermic Reactions: A Comprehensive Guide
Understanding the difference between exothermic and endothermic reactions is crucial for anyone studying chemistry, whether you're a high school student, a university undergraduate, or even a seasoned professional. These terms describe fundamental processes governing energy transfer in chemical and physical transformations, influencing everything from everyday occurrences to industrial processes. This comprehensive guide will delve into the core concepts, providing clear explanations, real-world examples, and practical tips to distinguish between these two crucial reaction types.
What are Exothermic Reactions?
Exothermic reactions are chemical or physical processes that release energy into their surroundings. This energy is typically released in the form of heat, but it can also manifest as light or sound. The prefix "exo" means "out," signifying the outward flow of energy. The key characteristic is that the system's energy decreases, and the surroundings gain energy. Think of it like this: the reaction is "giving off" energy.
Key Characteristics of Exothermic Reactions:
- Energy Release: The most defining feature. The products have lower energy than the reactants.
- Temperature Increase: Surrounding temperature increases as heat is released.
- Negative Enthalpy Change (ΔH): This is a crucial thermodynamic parameter. A negative ΔH indicates an exothermic reaction.
- Spontaneous Nature (Often): Many, but not all, exothermic reactions occur spontaneously, meaning they proceed without external input of energy. However, spontaneity is also influenced by entropy (disorder).
Examples of Exothermic Reactions:
Exothermic reactions are prevalent in everyday life and industrial processes. Here are some common examples:
- Combustion: Burning fuel, such as wood, gasoline, or natural gas, is a highly exothermic process that releases significant heat and light. This is the fundamental principle behind energy production in power plants and internal combustion engines.
- Neutralization Reactions: The reaction between an acid and a base, forming salt and water, often releases a considerable amount of heat. Think of the heat generated when mixing a strong acid like hydrochloric acid with a strong base like sodium hydroxide.
- Respiration: The biological process of respiration, where our bodies break down glucose to produce energy, is an exothermic reaction. This process releases heat, keeping our bodies warm.
- Explosions: Many explosions are incredibly rapid and exothermic reactions, releasing a tremendous amount of energy in a short time.
- Formation of Water: The simple reaction of hydrogen and oxygen to form water is highly exothermic, releasing a significant amount of heat.
Understanding Exothermic Reaction Diagrams:
Energy diagrams visually represent the energy changes during a reaction. For exothermic reactions, the energy of the products is lower than the energy of the reactants. The difference between these energies is the energy released during the reaction, often depicted as a negative ΔH value. The diagram usually shows an energy "hill" (activation energy) that needs to be overcome before the reaction proceeds, but the products ultimately sit at a lower energy level than the reactants.
What are Endothermic Reactions?
Endothermic reactions are the opposite of exothermic reactions. They absorb energy from their surroundings. The prefix "endo" signifies "within," implying that the system absorbs energy from its environment. In an endothermic reaction, the system's energy increases, and the surroundings lose energy.
Key Characteristics of Endothermic Reactions:
- Energy Absorption: The most crucial feature; the system absorbs energy from its surroundings.
- Temperature Decrease: The surrounding temperature decreases as the reaction absorbs heat.
- Positive Enthalpy Change (ΔH): A positive ΔH value is a definitive indicator of an endothermic reaction.
- Non-Spontaneous Nature (Often): Many endothermic reactions are not spontaneous and require an external energy source to proceed.
Examples of Endothermic Reactions:
Endothermic processes are also widespread, though they might not be as immediately apparent as exothermic ones.
- Melting Ice: Melting ice is a physical change, but it's an endothermic process. Energy (heat) is absorbed to break the bonds holding the water molecules in a solid state.
- Photosynthesis: Plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This is a critical endothermic reaction supporting almost all life on Earth.
- Cooking an Egg: Cooking an egg requires heat input to change the protein structure; it's an endothermic process.
- Dissolving Ammonium Nitrate in Water: Dissolving ammonium nitrate in water is a common endothermic reaction that leads to a significant drop in temperature. This is often used in instant cold packs.
- Electrolysis of Water: The decomposition of water into hydrogen and oxygen using electricity is an endothermic process.
Understanding Endothermic Reaction Diagrams:
The energy diagram for an endothermic reaction shows the products at a higher energy level than the reactants. The difference in energy represents the energy absorbed by the reaction (positive ΔH). Again, an activation energy barrier exists that needs to be overcome for the reaction to start, but the final energy state of the products is higher than the reactants.
Comparing Exothermic and Endothermic Reactions: A Table Summary
| Feature | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Change | Releases energy to the surroundings | Absorbs energy from the surroundings |
| ΔH (Enthalpy) | Negative (-) | Positive (+) |
| Temperature | Surrounding temperature increases | Surrounding temperature decreases |
| Spontaneity | Often spontaneous, but not always | Often non-spontaneous, requires energy input |
| Examples | Combustion, neutralization, respiration | Melting ice, photosynthesis, dissolving ammonium nitrate |
| Energy Diagram | Products at lower energy than reactants | Products at higher energy than reactants |
Practical Applications and Considerations:
The distinction between exothermic and endothermic reactions has significant practical implications across various fields:
- Industrial Processes: Understanding the energy changes involved is crucial for optimizing industrial chemical processes. For exothermic reactions, controlling the heat released is often essential to prevent safety hazards. For endothermic reactions, efficient energy input methods are needed.
- Materials Science: The design and development of new materials often involve carefully controlling exothermic and endothermic processes. For example, the curing of polymers often involves exothermic reactions, and understanding these reactions is vital for ensuring material stability.
- Medicine: Many biological processes are either exothermic or endothermic. Understanding these reactions is fundamental in areas such as drug design and development, where energy considerations play a crucial role in drug efficacy and metabolism.
- Environmental Science: Studying exothermic and endothermic reactions helps us comprehend natural processes like climate change. Many reactions involved in the carbon cycle are exothermic or endothermic, and understanding these energy exchanges is critical for predicting and mitigating climate change.
Further Exploration and Advanced Concepts:
- Entropy: While enthalpy (ΔH) describes the energy change, entropy (ΔS) describes the change in disorder. The Gibbs Free Energy (ΔG) combines both enthalpy and entropy to determine the spontaneity of a reaction: ΔG = ΔH - TΔS (where T is temperature). A negative ΔG indicates a spontaneous reaction.
- Activation Energy: This is the minimum energy required for a reaction to occur, regardless of whether the reaction is exothermic or endothermic. Catalysts can lower the activation energy, speeding up the reaction rate.
- Reaction Kinetics: This field studies the rates of chemical reactions, including the factors affecting the speed of both exothermic and endothermic processes.
By understanding the fundamental differences between exothermic and endothermic reactions and the factors influencing them, you gain a powerful tool for comprehending a wide range of chemical and physical phenomena. This knowledge is essential for various scientific and technological advancements. This comprehensive guide serves as a starting point for a deeper exploration into this fascinating area of chemistry.
Latest Posts
Latest Posts
-
New Delhi Delhi India Latitude Longitude
Mar 19, 2025
-
How To Convert Ratios To Percentages
Mar 19, 2025
-
What Is The Difference Between Percentile And Percent
Mar 19, 2025
-
Cometh The Hour Cometh The Man
Mar 19, 2025
-
What Is The Fraction For 20 Percent
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Differentiate Between Exothermic And Endothermic Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
