Differentiate Between Chemical Reaction And Nuclear Reaction
Juapaving
Mar 28, 2025 · 6 min read
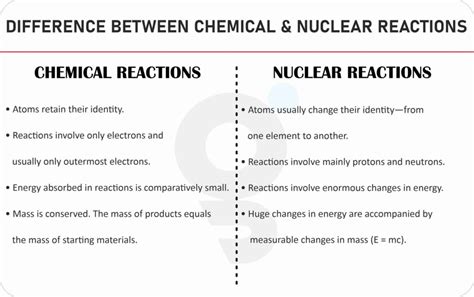
Table of Contents
Differentiating Chemical Reactions and Nuclear Reactions: A Comprehensive Guide
Understanding the fundamental differences between chemical reactions and nuclear reactions is crucial for comprehending the world around us, from the everyday processes happening in our bodies to the immense power harnessed in nuclear power plants. While both involve transformations of matter, they operate at vastly different levels and involve different types of forces and energy scales. This comprehensive guide will delve into the core distinctions, highlighting key differences and providing illustrative examples.
Defining Chemical Reactions
A chemical reaction is a process that involves the rearrangement of atoms within molecules or compounds to form new substances. This rearrangement involves breaking and forming chemical bonds, which are the forces that hold atoms together within molecules. Crucially, the identities of the atomic nuclei remain unchanged throughout a chemical reaction. Only the arrangement of electrons and the bonding between atoms are altered.
Key Characteristics of Chemical Reactions:
- Involve valence electrons: Chemical reactions primarily involve the interactions of valence electrons—the electrons in the outermost shell of an atom. These electrons participate in the formation and breaking of chemical bonds.
- Relatively low energy changes: The energy changes associated with chemical reactions are relatively small compared to nuclear reactions. This energy is often manifested as heat, light, or electricity.
- No change in atomic nuclei: The nuclei of the atoms involved remain unchanged. The same atoms are present before and after the reaction, just arranged differently.
- Observable changes: Chemical reactions often produce observable changes, such as color changes, gas evolution, precipitation, or temperature changes.
- Rates influenced by external factors: The rate of a chemical reaction can be influenced by factors such as temperature, pressure, concentration of reactants, and the presence of catalysts.
Examples of Chemical Reactions:
- Combustion: The burning of wood or fuel, which involves a rapid reaction with oxygen to produce carbon dioxide and water.
- Rusting: The slow reaction of iron with oxygen and water to form iron oxide (rust).
- Photosynthesis: The process by which plants convert light energy into chemical energy in the form of glucose.
- Digestion: The breakdown of food molecules into smaller molecules through a series of chemical reactions.
- Acid-base reactions: The reaction between an acid and a base, resulting in the formation of a salt and water.
Defining Nuclear Reactions
A nuclear reaction, on the other hand, involves changes within the atomic nucleus. These changes can result in the transformation of one element into another, a process known as transmutation. Nuclear reactions involve forces much stronger than the electromagnetic forces governing chemical bonds; these are the strong and weak nuclear forces, which are responsible for holding the protons and neutrons together in the nucleus.
Key Characteristics of Nuclear Reactions:
- Involve the nucleus: Nuclear reactions involve changes in the nucleus of an atom, affecting the number of protons and/or neutrons.
- Extremely high energy changes: Nuclear reactions involve enormous energy changes compared to chemical reactions. This energy is released as kinetic energy of particles, gamma radiation, or other forms of radiation.
- Transmutation of elements: Nuclear reactions often result in the formation of new elements, as the number of protons in the nucleus changes.
- Radiation emission: Nuclear reactions often produce various forms of ionizing radiation, such as alpha particles, beta particles, and gamma rays. These emissions can be harmful to living organisms.
- Rates generally not easily influenced: The rate of a nuclear reaction is generally less affected by external factors like temperature or pressure, compared to chemical reactions.
Examples of Nuclear Reactions:
- Nuclear fission: The splitting of a heavy atomic nucleus (like uranium or plutonium) into smaller nuclei, releasing a large amount of energy. This is the process used in nuclear power plants and atomic bombs.
- Nuclear fusion: The combining of light atomic nuclei (like hydrogen isotopes deuterium and tritium) to form a heavier nucleus (like helium), also releasing a vast amount of energy. This is the process that powers the sun and other stars.
- Radioactive decay: The spontaneous emission of particles or energy from an unstable atomic nucleus. This process can transform one element into another. Examples include alpha decay, beta decay, and gamma decay.
- Nuclear bombardment: The artificial transmutation of elements through bombardment of nuclei with particles like neutrons or protons. This is used in various applications, including producing medical isotopes.
A Comparative Table: Chemical vs. Nuclear Reactions
| Feature | Chemical Reaction | Nuclear Reaction |
|---|---|---|
| Location | Involves valence electrons and chemical bonds | Involves the atomic nucleus |
| Energy Change | Relatively small | Extremely large |
| Atomic Nuclei | Remain unchanged | Can change, resulting in transmutation |
| Elements | Elements remain the same | Elements can change |
| Radiation | No radiation emitted | Often emits ionizing radiation (alpha, beta, gamma) |
| Rate Affected by | Temperature, pressure, concentration, catalysts | Less affected by external factors |
| Bonds Involved | Chemical bonds (covalent, ionic, metallic) | Strong and weak nuclear forces |
| Examples | Combustion, rusting, photosynthesis, digestion | Fission, fusion, radioactive decay, nuclear bombardment |
Further Distinctions and Applications
The differences between chemical and nuclear reactions extend beyond the simple comparison above. Let's delve into some more nuanced distinctions and their practical applications:
Energy Scales:
The energy released in a nuclear reaction is several orders of magnitude greater than that of a chemical reaction. For instance, the energy released by burning a kilogram of coal is far less than the energy released by the fission of a kilogram of uranium. This tremendous energy difference is what makes nuclear reactions so powerful but also potentially dangerous.
Types of Forces:
Chemical reactions are governed by the electromagnetic force, the force of attraction or repulsion between charged particles. In contrast, nuclear reactions involve the strong and weak nuclear forces, which are much stronger than the electromagnetic force but act only over very short distances within the nucleus.
Applications:
The vast difference in energy levels leads to vastly different applications. Chemical reactions are the basis of most industrial processes, powering vehicles, generating electricity (combustion), and providing the energy for our bodies (metabolism). Nuclear reactions, on the other hand, are utilized in nuclear power plants for electricity generation, in nuclear medicine for diagnosis and treatment (radioisotopes), and in various scientific research areas.
Waste Products:
Chemical reactions generally produce waste products that are relatively benign and can be easily managed. Nuclear reactions, however, can produce radioactive waste that remains hazardous for thousands of years, posing significant challenges for long-term storage and disposal.
Controllability:
Chemical reactions are generally easy to control and stop, whereas nuclear reactions, especially fission reactions, require sophisticated control mechanisms to prevent runaway reactions and potential accidents.
Conclusion:
The differences between chemical and nuclear reactions are profound and far-reaching. Understanding these differences is essential for comprehending various phenomena in the natural world and for harnessing the power of both chemical and nuclear processes for beneficial purposes. While chemical reactions involve rearrangements of atoms within molecules, governed by electromagnetic forces, nuclear reactions involve changes within the atomic nucleus, governed by the strong and weak nuclear forces. These fundamental differences dictate the energy scales, the types of products formed, the potential applications, and the safety considerations associated with each type of reaction. By appreciating these distinctions, we can better understand and utilize the transformative power of both chemical and nuclear processes responsibly and effectively.
Latest Posts
Latest Posts
-
Is Butter Melting A Chemical Or Physical Change
Mar 31, 2025
-
Which Of The Following Is Correctly Matched A
Mar 31, 2025
-
The Temperature At Which A Solid Becomes A Liquid
Mar 31, 2025
-
How Many Pairs Of Homologous Chromosomes Do Females Have
Mar 31, 2025
-
54 As Product Of Prime Factors
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Differentiate Between Chemical Reaction And Nuclear Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
