Depression In Freezing Point Is A Colligative Property
Juapaving
Mar 17, 2025 · 6 min read
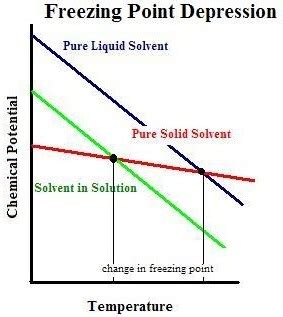
Table of Contents
Depression in Freezing Point: A Colligative Property Explained
Depression in freezing point, a crucial concept in chemistry, refers to the phenomenon where the freezing point of a solvent decreases when a solute is added. This isn't some arbitrary quirk of nature; it's a direct consequence of a class of properties called colligative properties, which depend solely on the number of solute particles present in a solution, not their identity. Understanding this principle unlocks a deeper understanding of solutions and their behavior. This article will delve into the intricacies of freezing point depression, exploring its mechanism, applications, and practical implications.
Understanding Colligative Properties
Before diving into the specifics of freezing point depression, it's essential to grasp the broader concept of colligative properties. These properties are characteristics of a solution that depend on the concentration of solute particles, regardless of the solute's nature. The four primary colligative properties are:
- Vapor pressure lowering: The presence of a non-volatile solute reduces the vapor pressure of the solvent.
- Boiling point elevation: The boiling point of a solution is higher than that of the pure solvent.
- Freezing point depression: The freezing point of a solution is lower than that of the pure solvent (our primary focus).
- Osmotic pressure: The pressure required to prevent the flow of solvent across a semipermeable membrane.
These properties arise because the solute particles interfere with the solvent's ability to transition between phases (solid, liquid, gas). In essence, they disrupt the equilibrium between phases.
The Mechanism of Freezing Point Depression
The freezing point of a pure substance is the temperature at which its solid and liquid phases coexist in equilibrium. At this point, the rate of freezing (liquid to solid) equals the rate of melting (solid to liquid). Introducing a solute disrupts this equilibrium by interfering with the solvent's ability to form a well-ordered solid structure (like a crystal lattice).
Imagine pure water freezing. Water molecules form a crystalline structure with strong hydrogen bonds. Adding a solute, like salt (NaCl), introduces ions (Na⁺ and Cl⁻) that interfere with this organized structure. The solute particles get in the way of the water molecules aligning themselves properly for crystallization. Consequently, a lower temperature is required for the water molecules to overcome this interference and form a solid structure. This results in a lower freezing point for the solution.
The Role of Solute Particles
It's crucial to emphasize that the magnitude of freezing point depression directly correlates with the number of solute particles, not their type. A solution containing 1 mole of glucose (a non-electrolyte) will exhibit a different freezing point depression compared to a solution containing 1 mole of sodium chloride (NaCl, an electrolyte). This difference arises from the dissociation of electrolytes.
Glucose, being a non-electrolyte, remains as a single molecule in solution. However, NaCl dissociates into two ions (Na⁺ and Cl⁻) per formula unit. This means that a 1 molal solution of NaCl will have twice the number of solute particles as a 1 molal solution of glucose. Therefore, the freezing point depression in the NaCl solution will be approximately twice that of the glucose solution.
This is quantified by the van't Hoff factor (i), which represents the number of particles a solute dissociates into in solution. For non-electrolytes, i ≈ 1. For NaCl, i ≈ 2. For more complex electrolytes like magnesium chloride (MgCl₂), i ≈ 3. The van't Hoff factor isn't always a whole number because of ion pairing and other complexities in solution chemistry.
The Formula for Freezing Point Depression
The magnitude of freezing point depression (ΔT<sub>f</sub>) can be calculated using the following equation:
ΔT<sub>f</sub> = i × K<sub>f</sub> × m
Where:
- ΔT<sub>f</sub> is the change in freezing point (in °C or K).
- i is the van't Hoff factor.
- K<sub>f</sub> is the cryoscopic constant (a solvent-specific constant). It represents the freezing point depression caused by a 1 molal solution of a non-electrolyte.
- m is the molality of the solution (moles of solute per kilogram of solvent).
The cryoscopic constant (K<sub>f</sub>) is a characteristic property of the solvent. For example, the K<sub>f</sub> for water is 1.86 °C/m. This means that a 1 molal solution of a non-electrolyte in water will have its freezing point lowered by approximately 1.86 °C.
Applications of Freezing Point Depression
The principle of freezing point depression has several important practical applications:
-
De-icing roads and pavements: Salt (NaCl) is commonly used to lower the freezing point of water, preventing ice formation on roads and runways during winter. The salt dissolves in the thin layer of water on the surface, lowering its freezing point below the ambient temperature.
-
Antifreeze in car radiators: Ethylene glycol is a common component of antifreeze solutions used in car radiators. It lowers the freezing point of water, preventing the coolant from freezing in cold climates and damaging the engine.
-
Food preservation: Freezing food at low temperatures slows down or stops the growth of microorganisms, preventing spoilage. The addition of solutes to the food can further lower the freezing point, achieving even lower temperatures for preservation.
-
Determination of molar mass: The freezing point depression method can be used to determine the molar mass of an unknown solute. By measuring the freezing point depression of a solution with a known mass of solute, the molality and subsequently the molar mass can be calculated.
-
Understanding biological systems: Freezing point depression plays a role in various biological systems. For example, the freezing point of body fluids is slightly lower than that of pure water, preventing freezing in cold environments.
Limitations and Considerations
While the freezing point depression equation provides a useful approximation, several factors can influence its accuracy:
-
Non-ideality of solutions: At high concentrations, solutions deviate from ideal behavior, and the van't Hoff factor might not be accurate. Ion pairing and other intermolecular interactions can affect the effective number of solute particles.
-
Association and dissociation: Some solutes associate or dissociate to a greater or lesser extent depending on concentration and temperature, affecting the effective van't Hoff factor.
-
Solubility limitations: The solute must be soluble in the solvent to effectively lower the freezing point.
-
Experimental errors: Accurate measurement of temperature and concentration is essential for obtaining reliable results.
Conclusion
Freezing point depression is a fundamental colligative property with significant theoretical and practical implications. Understanding its mechanism and applications allows us to appreciate the interplay between solute and solvent in solutions. From de-icing roads to preserving food to determining molar mass, this phenomenon demonstrates the power of basic chemical principles in solving real-world problems. Further research into the nuances of solution chemistry, particularly regarding non-ideal solutions and the complexities of the van't Hoff factor, will continue to refine our understanding and expand the applicability of this important concept. The inherent simplicity of the underlying principle, coupled with its wide-ranging impact, solidifies freezing point depression's importance in various scientific and engineering fields.
Latest Posts
Latest Posts
-
What Happens If Meiosis Does Not Occur
Mar 17, 2025
-
Least Common Multiple Of 8 12 15
Mar 17, 2025
-
What Is The Least Common Multiple Of 24 And 12
Mar 17, 2025
-
Which Of The Following Is Not Associated With Animal Cells
Mar 17, 2025
-
How To Find The Inverse Of A Relation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Depression In Freezing Point Is A Colligative Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
