Density Of Iron In Kg M3
Juapaving
Mar 10, 2025 · 6 min read
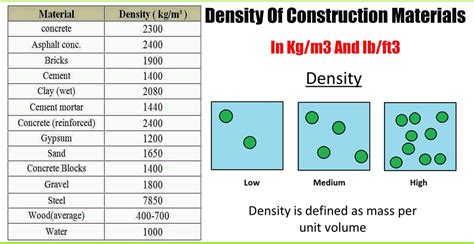
Table of Contents
Density of Iron in kg/m³: A Comprehensive Guide
Iron, a ubiquitous element crucial to human civilization and biological processes, boasts a remarkably consistent density. Understanding this density, expressed in kilograms per cubic meter (kg/m³), is vital across numerous scientific and engineering disciplines. This article delves into the intricacies of iron's density, exploring its variations, measurement techniques, applications, and the factors influencing its value.
What is Density?
Before focusing on iron specifically, let's establish a fundamental understanding of density. Density is a measure of mass per unit volume. In simpler terms, it tells us how much matter is packed into a given space. The formula for density is:
Density = Mass / Volume
The standard unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also commonly used. It's important to note that density is a intensive property, meaning it doesn't depend on the amount of substance present. A kilogram of iron will have the same density as a tonne of iron.
Density of Iron: The Standard Value
The generally accepted density of pure iron at room temperature (approximately 20°C or 68°F) and standard atmospheric pressure is approximately 7870 kg/m³. However, this is an average value, and slight variations can occur due to several factors discussed in the following sections. This value is crucial for numerous calculations in various fields, from structural engineering to material science.
Variations in Density: Factors to Consider
Several factors can subtly alter the measured density of iron:
-
Temperature: Like most materials, iron's density decreases with increasing temperature. The thermal expansion of iron causes the same mass to occupy a larger volume, resulting in a lower density. Precise temperature control is therefore critical when measuring iron's density.
-
Purity: The presence of impurities, even in small amounts, can significantly affect the density. Alloying iron with other elements, such as carbon (to create steel), alters its crystal structure and consequently its density. Steel, for instance, generally has a slightly lower density than pure iron due to the addition of carbon and other alloying elements. The specific density of a steel alloy depends heavily on its precise composition.
-
Crystal Structure: Iron exists in different crystalline forms (allotropes) depending on the temperature. These different allotropes have slightly different atomic arrangements, influencing the overall density. The most common allotrope at room temperature is body-centered cubic (BCC), but at higher temperatures, it transforms into face-centered cubic (FCC). These structural changes affect the packing efficiency of atoms, and hence the density.
-
Pressure: Applying external pressure compresses the iron atoms, decreasing the volume and thus increasing the density. This effect is more pronounced at higher pressures. However, under normal conditions, the impact of atmospheric pressure on iron's density is negligible.
-
Porosity: In the case of cast iron or other iron-based materials with a porous structure, the density will be lower than that of a solid, dense piece of pure iron. The presence of voids and air pockets within the material reduces its overall mass per unit volume.
Measurement Techniques for Determining Density
Several methods exist for accurately determining the density of iron, each with its own advantages and disadvantages:
-
Archimedes' Principle (Water Displacement): This classic method involves submerging a known mass of iron in water and measuring the volume of water displaced. By applying Archimedes' principle (the buoyant force is equal to the weight of the displaced fluid), the volume of the iron can be calculated, and consequently its density. This method is relatively simple but can be less precise for irregularly shaped samples.
-
Pycnometry: Pycnometry is a more precise technique involving using a precisely calibrated pycnometer (a special density bottle) to determine the volume of a known mass of iron. This method is particularly useful for accurately measuring the density of powders or irregularly shaped samples.
-
X-ray Diffraction: This sophisticated method uses X-ray diffraction patterns to determine the crystal structure and lattice parameters of iron. From these parameters, the atomic volume and consequently the density can be precisely calculated. This is a highly accurate technique but requires specialized equipment.
-
Computed Tomography (CT) Scanning: CT scanning provides a detailed 3D image of the internal structure of the iron sample. By analyzing the image, the volume and mass can be determined, allowing for the calculation of density, particularly useful for complex or porous samples.
Applications of Iron's Density Knowledge
The knowledge of iron's density is crucial in various applications:
-
Structural Engineering: Density is critical for calculating the weight and stress on structures made of iron or steel. Accurate density values are essential for designing bridges, buildings, and other load-bearing structures.
-
Material Science: Density is a key property considered in material selection for various applications. Engineers and scientists select materials based on their density, strength, and other properties to optimize performance and minimize weight.
-
Fluid Mechanics: The density of iron plays a crucial role in calculating the buoyancy and behavior of iron objects submerged in fluids. This is important in naval architecture and offshore engineering.
-
Geophysics: The density of iron is a fundamental parameter used in geophysical models to understand the Earth's internal structure and composition. Iron constitutes a significant portion of the Earth's core, and its density is crucial for understanding the planet's gravitational field and magnetic field.
-
Metallurgy: Accurate density measurements are essential for quality control in iron and steel production. Density can indicate the presence of impurities or variations in the material's composition.
-
Chemical Engineering: Density is used in various calculations in chemical processes involving iron or iron compounds. Accurate density data is crucial for designing and optimizing reactors and separation processes.
Conclusion: The Significance of Understanding Iron's Density
The density of iron, a seemingly simple physical property, plays a pivotal role in various scientific and engineering disciplines. Understanding the factors that influence its value and the methods for its precise measurement is crucial for designing safe and efficient structures, materials, and processes. While the standard value of 7870 kg/m³ serves as a useful approximation, recognizing the potential variations due to temperature, purity, and other factors ensures greater accuracy in applications where precision is paramount. The continued exploration and refinement of density measurement techniques will undoubtedly contribute to further advances in numerous fields relying on the properties of this essential metal. As research continues, our understanding of iron's density and its subtle variations will undoubtedly deepen, leading to even more sophisticated applications and innovations. This comprehensive understanding is essential for optimizing designs, enhancing safety, and driving progress across numerous sectors that rely on the unparalleled properties of iron and its alloys.
Latest Posts
Latest Posts
-
What Are The Factors Of 77
Mar 10, 2025
-
What Is A Plan In Geography
Mar 10, 2025
-
Addition And Subtraction Of Rational Algebraic Expressions Calculator
Mar 10, 2025
-
Whats The Lcm Of 7 And 4
Mar 10, 2025
-
3 Ways To Dissolve Something Faster
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Density Of Iron In Kg M3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
